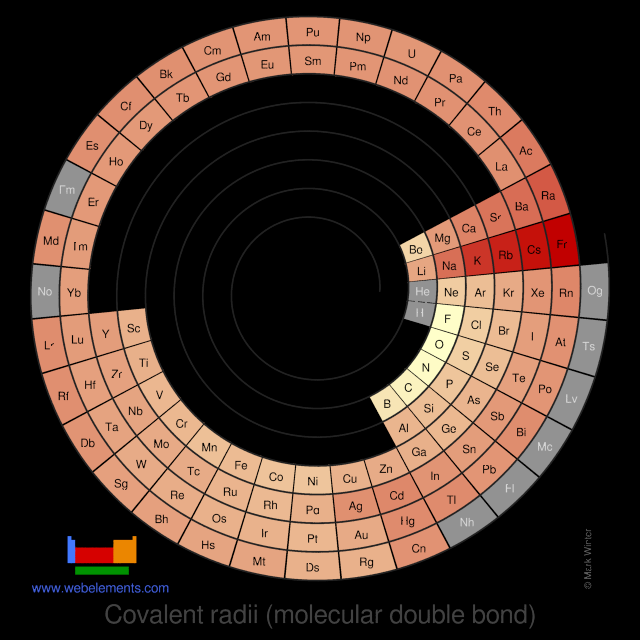
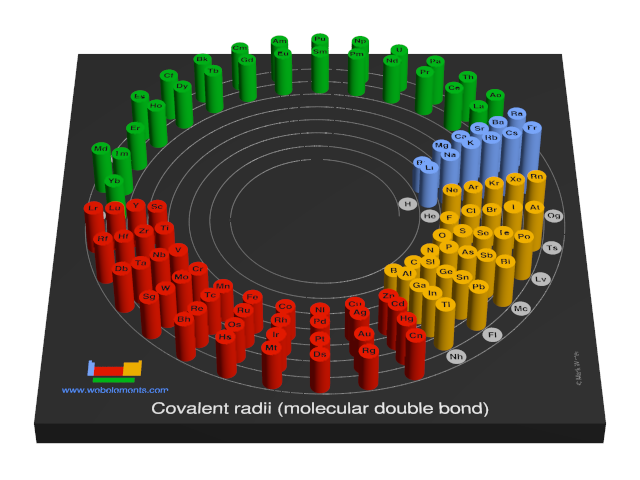
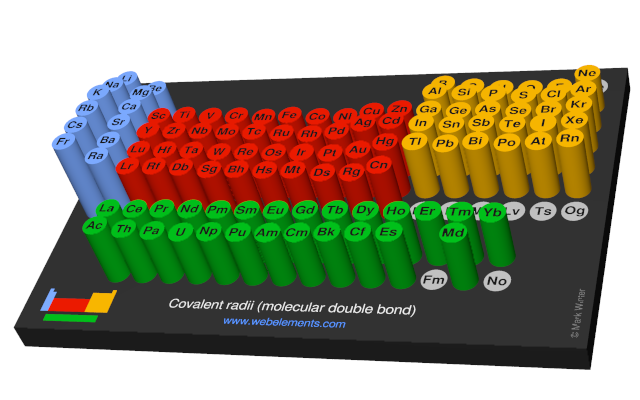
Covalent radii (molecular double bond)
This gives double-bond covalent radii using specific group valencies and oxidation numbers.


Units
pm
Notes
This is a self-consistent system of additive covalent radii, R(AB)=r(A) + r(B), set up for the periodic table, Z = 3+112. The primary bond lengths, R, are taken from experimental or theoretical data corresponding to chosen group valencies. All elements and all admitted data points are treated as equal. Radii were then obtained self-consistently through a least-squares fit. Specific molecular coordination numbers and oxidation states are chosen for each element involved.Select from the following links to see visual periodicity representations for atomic radii, covalent radii, and van der Waals radii. Ionic radii are also available.
- Atomic radii
- Atomic radii (empirical)
- Atomic radii (absolute)
- Atomic radii (density cutoff)
- Covalent radii
- Covalent radii revisited (2008 values)
- Covalent radii (molecular single bond)
- Covalent radii (molecular double bond)
- Covalent radii (molecular triple bond)
- Van der Waals radius
Literature sources
- P. Pyykkö and M. Atsumi, Molecular Double-Bond Covalent Radii for Elements LiÐE112, Chem. Eur. J., 2009, 15, 12770-12779.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||