Zirconium - 40Zr: the essentials
- Name: zirconium
- Symbol: Zr
- Atomic number: 40
- Relative atomic mass (Ar): 91.224 (2) g
- Standard state: solid at 298 K
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table: 4
- Group name: (none)
- Period in periodic table: 5
- Block in periodic table: d
- Shell structure: 2.8.18.10.2
- CAS Registry: 7440-67-7
Zirconium atoms have 40 electrons and the shell structure is 2.8.18.10.2. The ground state electronic configuration of neutral zirconium is [Kr].4d2.5s2 and the term symbol of zirconium is 3F2.
Zirconium: description
Zirconium is a greyish-white lustrous metal. The finely divided metal can ignite spontaneously in air, especially at elevated temperatures. The solid metal is much more difficult to ignite. The inherent toxicity of zirconium compounds is low. Hafnium is invariably found in zirconium ores, and the separation is difficult. Commercial grade zirconium contains from 1 to 3% hafnium. The hafnium is removed from the zirconium used in the nuclear power industry.
Zirconium is found in S-type stars, and has been identified in the sun and meteorites. Analyses of lunar rock samples show a surprisingly high zirconium oxide content as compared with terrestrial rocks. Some forms of zircon (ZrSiO4) have excellent gemstone qualities.

The image above is a virtual representation of zirconium metal calculated by Patrick Callet using the complex diectric function of the element only.

Zirconium foil.
Zirconium: physical properties
Density of solid: 6511 kg m-3
Molar volume: 14.02 cm3
Thermal conductivity: 22.7 W m‑1 K‑1
Zirconium: heat properties
Melting point: 2128 [1855 °C (3371 °F)] K
Boiling point: 4682 [4409 °C (7968 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Zirconium: atom sizes
Atomic radius (empirical): 155 pm
Molecular single bond covalent radius: 154 (coordination number 4) ppm
van der Waals radius: 252 ppm
Zirconium: electronegativities
Pauling electronegativity: 1.33 (Pauling units)
Allred Rochow electronegativity: 1.22 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Zirconium: orbital properties
First ionisation energy: 640.10 kJ mol‑1
Second ionisation energy: 1267 kJ mol‑1
Third ionisation energy: 2236 kJ mol‑1
Zirconium: abundances
Universe: 50 ppb by weight
Crustal rocks: 130000 ppb by weight
Human: 50 ppb by weight
Zirconium: crystal structure
Zirconium: biological data
Human abundance by weight: 50 ppb by weight
Zirconium has no biological role. The tolerance of human tissues to it makes the metal suitable for some artificial joints and limbs.
Zirconium: uses
Zirconium: reactions
Reactions of zirconium as the element with air, water, halogens, acids, and bases where known.
Zirconium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of zirconium where known.
Zirconium: compound properties
Bond strengths; lattice energies of zirconium halides, hydrides, oxides (where known); and reduction potentials where known.
Zirconium: history
Zirconium was discovered by Martin Heinrich Klaproth in 1789 at Berlin, Germany. Origin of name: from the Arabic word "zargun" meaning "gold colour".Zirconium: isotopes
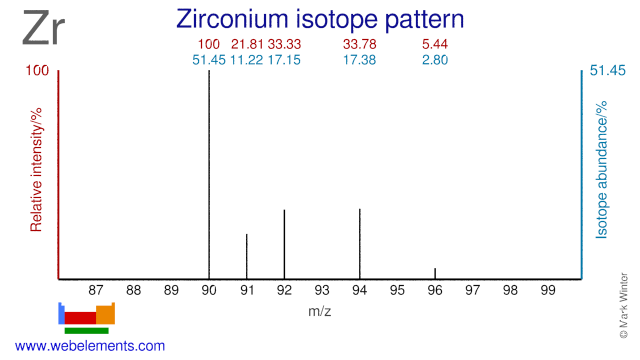
Zirconium has five stable isotopes, of which a few are used for the production of radioisotopes. Although radioactive Zr-95 is a fission product, it can also produced by neutron irradiation of Zr-94. Zr-96 has been used for the production of the radioisotope Zr-97. Zr-90 can be used for the production of the PET isotope Nb-90. Finally, Zr-90 has been proposed for cladding in nuclear fuel. The use of Zr-90 would lower even further the already low neutron absorption cross section of natural Zr that is currently used as fuel cladding.
Zirconium: isolation
Isolation: zirconium is available from commercial sources so preparation in the laboratory is not normally required. In industry, reduction of ores with carbon is not a useful option as intractable carbides are produced. As for titanium, the Kroll method is used for zirconium and involves the action of chlorine and carbon upon baddeleyite (ZrO2). The resultant zirconium tetrachloride, ZrCl4, is separated from the iron trichloride, FeCl3, by fractional distillation. Finally ZrCl4 is reduced to metallic zirconium by reduction with magnesium, Mg. Air is excluded so as to prevent contamination of the product with oxygen or nitrogen.
ZrO2 + 2Cl2 + 2C (900°C) → ZrCl4 + 2CO
ZrCl4 + 2Mg (1100°C) → 2MgCl2 + Zr
Excess magensium and magnesium dichloride is removed from the product by treatment with water and hydrochloric acid to leave a zirconium "sponge". This can be melted under helium by electrical heating.