Europium - 63Eu: the essentials
- Name: europium
- Symbol: Eu
- Atomic number: 63
- Relative atomic mass (Ar): 151.964 (1) g [see note g]
- Standard state: solid at 298 K
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table:
- Group name: Lanthanoid
- Period in periodic table: 6 (lanthanoid)
- Block in periodic table: f
- Shell structure: 2.8.18.25.8.2
- CAS Registry: 7440-53-1
Europium atoms have 63 electrons and the shell structure is 2.8.18.25.8.2. The ground state electronic configuration of neutral europium is [Xe].4f7.6s2 and the term symbol of europium is 8S7/2.
Europium: description
Europium ignites in air at about 150 to 180°C. Europium is about as hard as lead and is quite ductile. It is the most reactive of the rare earth metals, quickly oxidising in air. It resembles calcium in its reaction with water. It is used in television screens to produce a red colour.

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.
Europium: physical properties
Density of solid: 5244 kg m-3
Molar volume: 28.97 cm3
Thermal conductivity: 14 W m‑1 K‑1
Europium: heat properties
Melting point: 1099 [826 °C (1519 °F)] K
Boiling point: 1800 [1527 °C (2781 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Europium: atom sizes
Atomic radius (empirical): 185 pm
Molecular single bond covalent radius: 168 (coordination number 3) ppm
van der Waals radius: 283 ppm
Europium: electronegativities
Pauling electronegativity: (no data) (Pauling units)
Allred Rochow electronegativity: 1.01 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Europium: orbital properties
First ionisation energy: 547.11 kJ mol‑1
Second ionisation energy: 1084.5 kJ mol‑1
Third ionisation energy: 2400 kJ mol‑1
Europium: abundances
Universe: 0.5 ppb by weight
Crustal rocks: 1800 ppb by weight
Human: (no data) ppb by weight
Europium: crystal structure
Europium: biological data
Human abundance by weight: (no data) ppb by weight
Europium has no biological role.
Europium: uses
Europium: reactions
Reactions of europium as the element with air, water, halogens, acids, and bases where known.
Europium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of europium where known.
Europium: compound properties
Bond strengths; lattice energies of europium halides, hydrides, oxides (where known); and reduction potentials where known.
Europium: history
Europium was discovered by Eugene Demarcay in 1901 at France. Origin of name: named after "Europe".Europium: isotopes
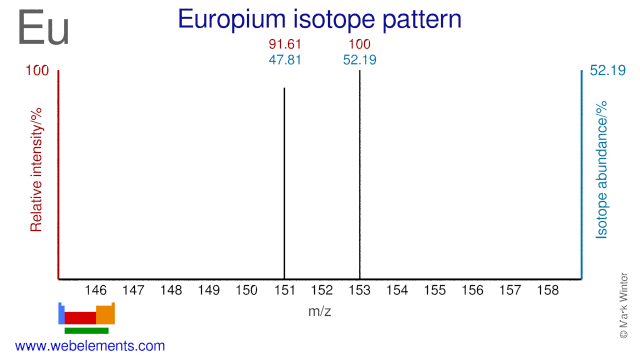
Europium has two stable isotopes and both are used for the production of radioisotopes. Eu-151 is used for the production of Eu-152 which is used as a reference source in gammaspectroscopy. Eu-153 can be used for the production of high specific activity Sm-153 via fast neutron irradiation.
Europium: isolation
Isolation: europium metal is available commercially so it is not normally necessary to make it in the laboratory, which is just as well as it is difficult to isolate as the pure metal. This is largely because of the way it is found in nature. The lanthanoids are found in nature in a number of minerals. The most important are xenotime, monazite, and bastnaesite. The first two are orthophosphate minerals LnPO4 (Ln deonotes a mixture of all the lanthanoids except promethium which is vanishingly rare) and the third is a fluoride carbonate LnCO3F. Lanthanoids with even atomic numbers are more common. The most comon lanthanoids in these minerals are, in order, cerium, lanthanum, neodymium, and praseodymium. Monazite also contains thorium and ytrrium which makes handling difficult since thorium and its decomposition products are radioactive.
For many purposes it is not particularly necessary to separate the metals, but if separation into individual metals is required, the process is complex. Initially, the metals are extracted as salts from the ores by extraction with sulphuric acid (H2SO4), hydrochloric acid (HCl), and sodium hydroxide (NaOH). Modern purification techniques for these lanthanoid salt mixtures are ingenious and involve selective complexation techniques, solvent extractions, and ion exchange chromatography.
Pure europium is available through the electrolysis of a mixture of molten EuCl3 and NaCl (or CaCl2) in a graphite cell which acts as cathode using graphite as anode. The other product is chlorine gas.