Neon - 10Ne: the essentials
- Name: neon
- Symbol: Ne
- Atomic number: 10
- Relative atomic mass (Ar): 20.1797 (6) g,m [see notes g m]
- Standard state: gas at 298 K
- Appearance: colourless
- Classification: Non-metallic
- Group in periodic table: 18
- Group name: Noble gas
- Period in periodic table: 2
- Block in periodic table: p
- Shell structure: 2.8
- CAS Registry: 7440-01-9
Neon atoms have 10 electrons and the shell structure is 2.8. The ground state electronic configuration of neutral neon is [He].2s2.2p6 and the term symbol of neon is 1S0.
Neon: description
Neon is a very inert element. Neon forms an unstable hydrate. In a vacuum discharge tube, neon glows reddish orange. Of all the rare gases, the discharge of neon is the most intense at ordinary voltages and currents. It is present in the atmosphere as 1 part in 65000.
Liquid neon has over 40 times more refrigerating capacity than liquid helium, and more than 3 times that of liquid hydrogen.
Neon: physical properties
Density of solid: 1444 kg m-3
Molar volume: 13.23 cm3
Thermal conductivity: 0.0491 W m‑1 K‑1
Neon: heat properties
Melting point: 24.56 [‑248.59 °C (‑415.46 °F)] K
Boiling point: 27.07 [‑246.08 °C (‑410.94 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Neon: atom sizes
Atomic radius (empirical): (no data) pm
Molecular single bond covalent radius: 67 (coordination number 1,2) ppm
van der Waals radius: [ 158 ] ppm
Neon: electronegativities
Pauling electronegativity: (no data) (Pauling units)
Allred Rochow electronegativity: 4.84 (Pauling units)
Mulliken-Jaffe electronegativity: 3.98 (12.5% s orbital)
Neon: orbital properties
First ionisation energy: 2080.66 kJ mol‑1
Second ionisation energy: 3952.32 kJ mol‑1
Third ionisation energy: 6119.42 kJ mol‑1
Neon: abundances
Universe: 1300000 ppb by weight
Crustal rocks: 3.0 ppb by weight
Human: (no data) ppb by weight
Neon: crystal structure
Neon: biological data
Human abundance by weight: (no data) ppb by weight
Neon has no biological role.
Neon: uses
Neon: reactions
Reactions of neon as the element with air, water, halogens, acids, and bases where known.
Neon: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of neon where known.
Neon: compound properties
Bond strengths; lattice energies of neon halides, hydrides, oxides (where known); and reduction potentials where known.
Neon: history
Neon was discovered by Sir William Ramsay, Morris W. Travers in 1898 at London, England. Origin of name: from the Greek word "neon" meaning "new".Neon: isotopes
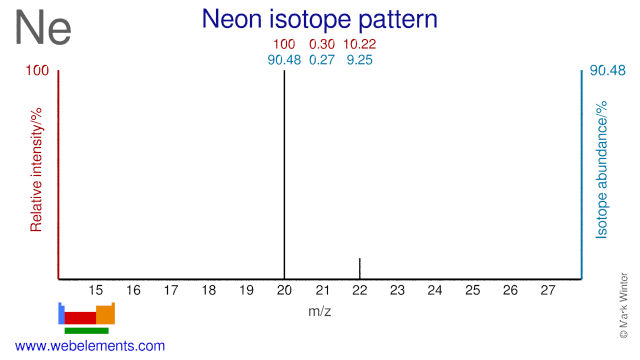
The three Neon isotopes are used for various purposes. Ne-22 is used for the production of the medical radioisotope Na-22. Ne-20 can be used for the production of F-18, although the route via O-18 is by far the most commonly used. Ne-21 has been used in Masers to study quantum physics.
Neon: isolation
Isolation: neon is present to a small extent in the atmosphere and is obtained as a byproduct from the liquefaction and separation of air. This would not normally be carried out in the laboratory and neon is available commercially in cylinders under pressure.