Thorium - 90Th: the essentials
- Name: thorium
- Symbol: Th
- Atomic number: 90
- Relative atomic mass (Ar): 232.0377 (4) [see note g]
- Standard state: solid at 298 K
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table:
- Group name: Actinoid
- Period in periodic table: 7 (actinoid)
- Block in periodic table: f
- Shell structure: 2.8.18.32.18.10.2
- CAS Registry: 7440-29-1
Thorium atoms have 90 electrons and the shell structure is 2.8.18.32.18.10.2. The ground state electronic configuration of neutral thorium is [Rn].6d2.7s2 and the term symbol of thorium is 3F2.
Thorium: description
Thorium is a source of nuclear power. There is probably more untapped energy available for use from thorium in the minerals of the earth's crust than from combined uranium and fossil fuel sources. Much of the internal heat the earth has been attributed to thorium and uranium.
When pure, thorium is a silvery white metal which is air-stable and retains its lustre for several months. When contaminated with the oxide, thorium slowly tarnishes in air, becoming grey and finally black. Thorium oxide has a melting point of 3300°C, the highest of all oxides. Only a few elements, such as tungsten, and a few compounds, such as tantalum carbide, have higher melting points.
Thorium is slowly attacked by water, but does not dissolve readily in most common acids, except hydrochloric. Powdered thorium metal is often pyrophoric and should be carefully handled.When heated in air, thorium turnings ignite and burn brilliantly with a white light.
Thorium is named for Thor, the Scandinavian god of war. It is found in thorite and thorianite in New England (USA) and other sites.

Image adapted with permission from Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Thorium: physical properties
Density of solid: 11724 kg m-3
Molar volume: 19.80 cm3
Thermal conductivity: 54 W m‑1 K‑1
Thorium: heat properties
Melting point: 2115 [1750 °C (3182 °F)] K
Boiling point: 5093 [4820 °C (8708 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Thorium: atom sizes
Atomic radius (empirical): 180 pm
Molecular single bond covalent radius: 175 (coordination number 4) ppm
van der Waals radius: [ 305 ] ppm
Thorium: electronegativities
Pauling electronegativity: 1.3 (Pauling units)
Allred Rochow electronegativity: 1.11 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Thorium: orbital properties
First ionisation energy: 608.50 kJ mol‑1
Second ionisation energy: 1167 kJ mol‑1
Third ionisation energy: 1768 kJ mol‑1
Thorium: abundances
Universe: 0.4 ppb by weight
Crustal rocks: 6000 ppb by weight
Human: (no data) ppb by weight
Thorium: crystal structure
Thorium: biological data
Human abundance by weight: (no data) ppb by weight
Thorium has no biological role.
Thorium: uses
Thorium: reactions
Reactions of thorium as the element with air, water, halogens, acids, and bases where known.
Thorium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of thorium where known.
Thorium: compound properties
Bond strengths; lattice energies of thorium halides, hydrides, oxides (where known); and reduction potentials where known.
Thorium: history
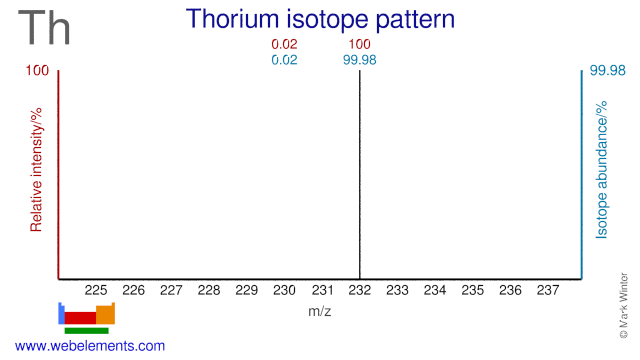
Thorium was discovered by Jöns Berzelius in 1829 at Sweden. Origin of name: named after "Thor", the mythological Scandinavian god of war.Thorium: isotopes
Thorium: isolation
Isolation: coming soon!