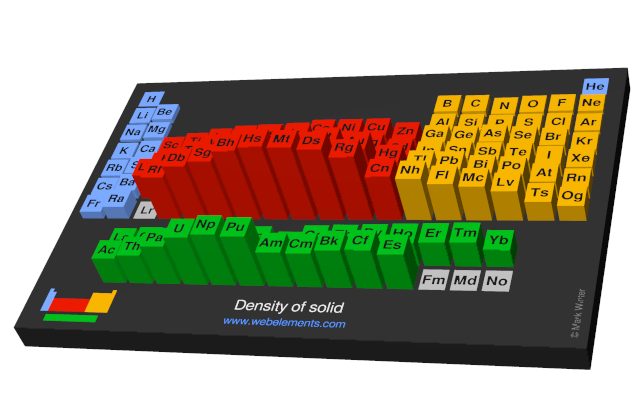
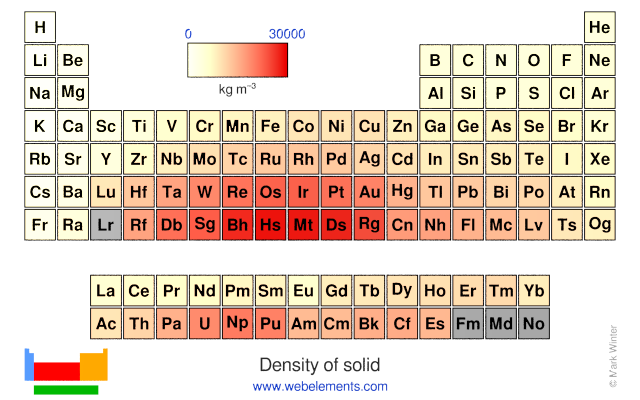
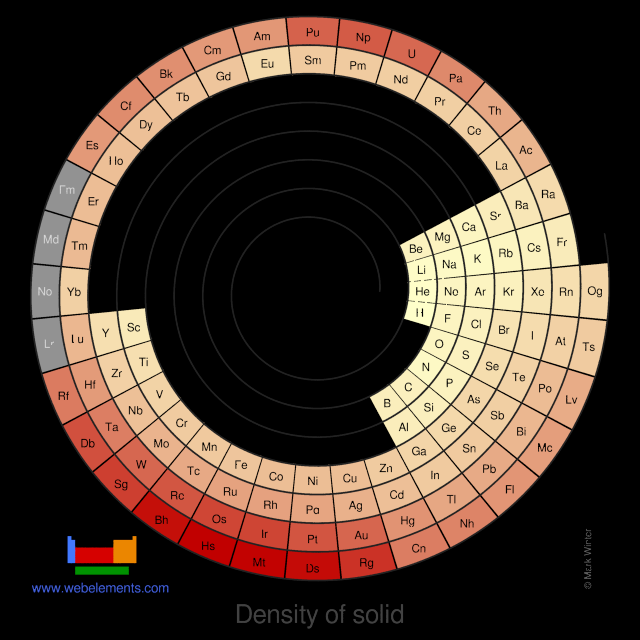
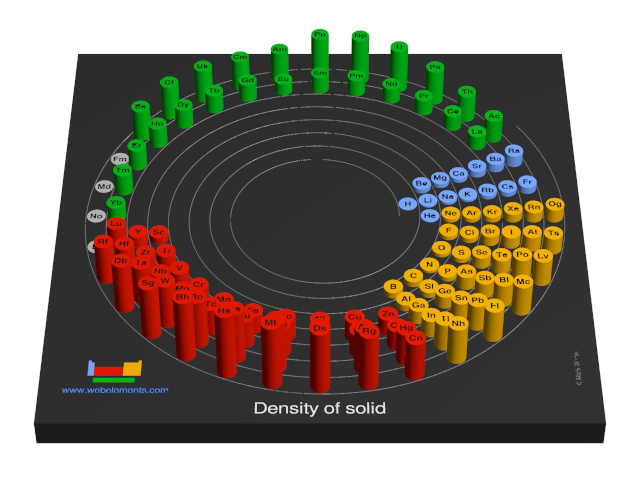
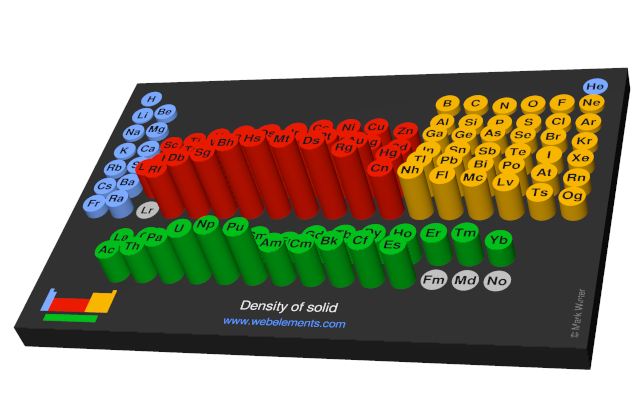
Density of solid
Density refers to the mass contained within a unit volume under specified conditions. Data given here refer to the solid. Density is temperature dependent and different allotopes of one element possess different densities. Where possible, values are given at or near ambient temperature, hopefully of the thermodynamically most favoured allotrope.
Units
kg m-3
Notes
The preferred units are kg m-3 because the basic SI unit of weight is the kilogramme (kg) and the preferred unit of length is the metre (m). To convert to the commonly used units of g cm-3, divide by 1000.
Literature sources
- A.M. James and M.P. Lord in Macmillan's Chemical and Physical Data, Macmillan, London, UK, 1992.
- D.R. Lide, (ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 77th edition, 1996.
- J.A. Dean (ed) in Lange's Handbook of Chemistry, McGraw-Hill, New York, USA, 14th edition, 1992.
- G.W.C. Kaye and T.H. Laby in Tables of physical and chemical constants, Longman, London, UK, 15th edition, 1993.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||