Vanadium - 23V: the essentials
- Name: vanadium
- Symbol: V
- Atomic number: 23
- Relative atomic mass (Ar): 50.9415 (1)
- Standard state: solid at 298 K
- Appearance: silvery grey metallic
- Classification: Metallic
- Group in periodic table: 5
- Group name: (none)
- Period in periodic table: 4
- Block in periodic table: d
- Shell structure: 2.8.11.2
- CAS Registry: 7440-62-2
Vanadium atoms have 23 electrons and the shell structure is 2.8.11.2. The ground state electronic configuration of neutral vanadium is [Ar].3d3.4s2 and the term symbol of vanadium is 4F3/2.
Vanadium: description
Pure vanadium is a greyish silvery metal, and is soft and ductile. It has good corrosion resistance to alkalis, sulphuric acid, hydrochloric acid, and salt waters. The metal oxidizes readily above 660°C to form V2O5. Industrially, most vanadium produced is used as an additive to improve steels.

Vanadium foil.
Vanadium: physical properties
Density of solid: 6110 kg m-3
Molar volume: 8.32 cm3
Thermal conductivity: 30.7 W m‑1 K‑1
Vanadium: heat properties
Melting point: 2183 [1910 °C (3470 °F)] K
Boiling point: 3680 [3407 °C (6165 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Vanadium: atom sizes
Atomic radius (empirical): 135 pm
Molecular single bond covalent radius: 134 (coordination number 5) ppm
van der Waals radius: 242 ppm
Vanadium: electronegativities
Pauling electronegativity: 1.63 (Pauling units)
Allred Rochow electronegativity: 1.45 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Vanadium: orbital properties
First ionisation energy: 650.91 kJ mol‑1
Second ionisation energy: 1412.0 kJ mol‑1
Third ionisation energy: 2828.09 kJ mol‑1
Vanadium: abundances
Universe: 1000 ppb by weight
Crustal rocks: 190000 ppb by weight
Human: 30 ppb by weight
Vanadium: crystal structure
Vanadium: biological data
Human abundance by weight: 30 ppb by weight
Vanadium is essential to sea squirts (ascidians). The concentration of vanadium in sea squirts is a million times higher than in sea water as a consequence of their ability to concentrate vanadium.
Vanadium is a necessary part of the diet of rats and chicks, but only is very small amounts. Deficiencies cause reduced growth and impair reproduction.
Vanadium: uses
Vanadium: reactions
Reactions of vanadium as the element with air, water, halogens, acids, and bases where known.
Vanadium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of vanadium where known.
Vanadium: compound properties
Bond strengths; lattice energies of vanadium halides, hydrides, oxides (where known); and reduction potentials where known.
Vanadium: history
Vanadium was discovered by Andres Manuel del Rio and Nils Sefström in 1801 at Mexico and Sweden. Origin of name: named after "Vanadis", the goddess of beauty in Scandinavian mythology.Vanadium: isotopes
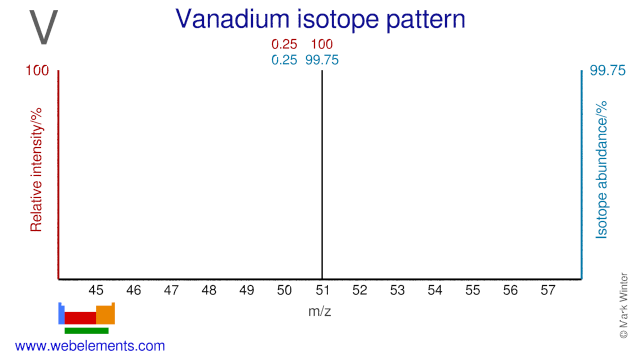
Vanadium has two isotopes and one of them, V-50, has a very low natural abundance (0.25%). V-50 is occasionally used for research purposes. V-51 is used in diabetes and metabolism studies.
Vanadium: isolation
Isolation: vanadium is available commercially and production of a sample in the laboratory is not normally required. Commercially, routes leading to matallic vanadium as main product are not usually required as enough is produced as byproduct in other processes.
In industry, heating of vanadium ore or residues from other processes with salt, NaCl, or sodium carbonate, Na2CO3, at about 850°C gives sodium vanadate, NaVO3. This is dissolved in water and acidified to give a red solid which in turn is melted to form a crude form of vanadium pentoxide, "V2O5". Reduction of vanadium pentoxide with calcium, Ca, gives pure vanadium. An alternative suitable for small scales is the reduction of vanadium pentachloride, VCl5, with hydrogen, H2, or magnesium, Mg. Many other methodsare also in use.
Industrially, most vanadium is used as an additive to improve steels. Rather than proceed via pure vanadium metal it is often sufficient to react the crude of vanadium pentoxide, "V2O5", with crude iron. This produces ferrovanadium suitable for further work.