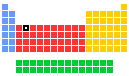
Titanium - 22Ti: the essentials
- Name: titanium
- Symbol: Ti
- Atomic number: 22
- Relative atomic mass (Ar): 47.867 (1)
- Standard state: solid at 298 K
- Appearance: silvery metallic
- Classification: Metallic
- Group in periodic table: 4
- Group name: (none)
- Period in periodic table: 4
- Block in periodic table: d
- Shell structure: 2.8.10.2
- CAS Registry: 7440-32-6
Titanium atoms have 22 electrons and the shell structure is 2.8.10.2. The ground state electronic configuration of neutral titanium is [Ar].3d2.4s2 and the term symbol of titanium is 3F2.
Titanium: description
Titanium s a lustrous, white metal when pure. Titanium minerals are quite common. The metal has a low density, good strength, is easily fabricated, and has excellent corrosion resistance. The metal burns in air and is the only element that burns in nitrogen. It is marvellous in fireworks.
Titanium is resistant to dilute sulphuric and hydrochloric acid, most organic acids, damp chlorine gas, and chloride solutions. Titanium metal is considered to be physiologically inert.
Titanium is present in meteorites and in the sun. Some lunar rocks contain high concentrations of the dioxide, TiO2. Titanium oxide bands are prominent in the spectra of M-type stars.

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.

The result from adding titanium powder to a burning mixture of potassium chlorate and sucrose (only to be demonstrated by a professionally qualified chemist).
Titanium: physical properties
Density of solid: 4507 kg m-3
Molar volume: 10.64 cm3
Thermal conductivity: 21.9 W m‑1 K‑1
Titanium: heat properties
Melting point: 1941 [1668 °C (3034 °F)] K
Boiling point: 3560 [3287 °C (5949 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Titanium: atom sizes
Atomic radius (empirical): 140 pm
Molecular single bond covalent radius: 136 (coordination number 4) ppm
van der Waals radius: 246 ppm
Titanium: electronegativities
Pauling electronegativity: 1.54 (Pauling units)
Allred Rochow electronegativity: 1.32 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Titanium: orbital properties
First ionisation energy: 658.81 kJ mol‑1
Second ionisation energy: 1309.84 kJ mol‑1
Third ionisation energy: 2652.55 kJ mol‑1
Titanium: abundances
Universe: 3000 ppb by weight
Crustal rocks: 6600000 ppb by weight
Human: (no data) ppb by weight
Titanium: crystal structure
Titanium: biological data
Human abundance by weight: (no data) ppb by weight
Titanium has no biological role. The metal is regarded as hypoallergenic.
Titanium: uses
Titanium: reactions
Reactions of titanium as the element with air, water, halogens, acids, and bases where known.
Titanium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of titanium where known.
Titanium: compound properties
Bond strengths; lattice energies of titanium halides, hydrides, oxides (where known); and reduction potentials where known.
Titanium: history
Titanium was discovered by William Gregor in 1791 at England. Origin of name: named after the "Titans", (the sons of the Earth goddess in Greek mythology).Titanium: isotopes
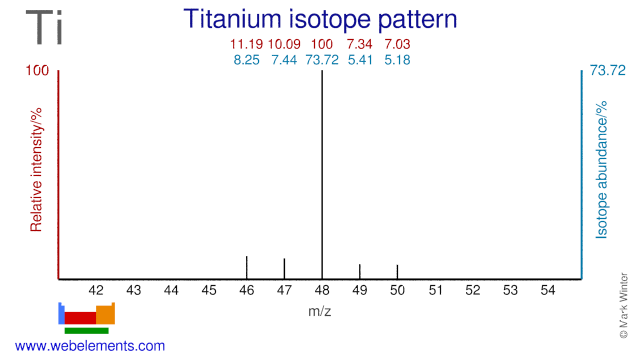
The five stable Titanium isotopes are used for a wide range of studies and applications. Ti-48 is used for the production of the radioisotope V-48 which is used in nutritional studies and for calibrating PET instrumentation. Ti-47 is occasionally used as an alternative precursor for the production of V-48. Ti-50 is used in the production of super heavy elements whereby Lead or Bismuth targets are bombarded with Ti-50. Finally, Ti-49 is used in the production of the radioisotope V-49.
Titanium: isolation
Isolation: titanium is readily available from commercial sources so preparation in the laboratory is not normally required. In industry, reduction of ores with carbon is not a useful option as intractable carbides are produced. The Kroll method is used on large scales and involves the action of chlorine and carbon upon ilmenite (TiFeO3) or rutile (TiO2). The resultant titanium tetrachloride, TiCl4, is separated from the iron trichloride, FeCl3, by fractional distillation. Finally TiCl4 is reduced to metallic titanium by reduction with magnesium, Mg. Air is excluded so as to prevent contamination of the product with oxygen or nitrogen.
2TiFeO3 + 7Cl2 + 6C (900°C) → 2TiCl4 + 2FeCl3 + 6CO
TiCl4 + 2Mg (1100°C) → 2MgCl2 + Ti
Excess magensium and magneium dichloride is removed from the product bytreatment with water and hydrochloric acid to leave a titanium "sponge". This can be melted under a helium or argon atmosphere to allow casting as bars.