Platinum - 78Pt: the essentials
- Name: platinum
- Symbol: Pt
- Atomic number: 78
- Relative atomic mass (Ar): 195.084 (9)
- Standard state: solid at 298 K
- Appearance: greyish white
- Classification: Metallic
- Group in periodic table: 10
- Group name: Precious metal or platinum group metal
- Period in periodic table: 6
- Block in periodic table: d
- Shell structure: 2.8.18.32.17.1
- CAS Registry: 7440-06-4
Platinum atoms have 78 electrons and the shell structure is 2.8.18.32.17.1. The ground state electronic configuration of neutral platinum is [Xe].4f14.5d9.6s1 and the term symbol of platinum is 3D3.
Platinum: description
Ruthenium, rhodium, palladium, osmium, iridium, and platinum together make up a group of elements referred to as the platinum group metals (PGM).
Platinum is a beautiful silvery-white metal, when pure, and is malleable and ductile. It has a coefficient of expansion almost equal to that of soda-lime-silica glass, and is therefore used to make sealed electrodes in glass systems.
The metal does not oxidise in air. It is insoluble in hydrochloric and nitric acid, but dissolves when they are mixed as aqua regia, forming chloroplatinic acid (H2PtCl6), an important compound. It is corroded by halogens, cyanides, sulphur and alkalis. Hydrogen and oxygen gas mixtures explode in the presence of platinum wire.

The above shows a platinum crucible.
Platinum: physical properties
Density of solid: 21090 kg m-3
Molar volume: 9.09 cm3
Thermal conductivity: 72 W m‑1 K‑1
Platinum: heat properties
Melting point: 2041.4 [1768.3 °C (3214.9 °F)] K
Boiling point: 4098 [3825 °C (6917 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Platinum: atom sizes
Atomic radius (empirical): 135 pm
Molecular single bond covalent radius: 123 (coordination number 3) ppm
van der Waals radius: 232 ppm
Platinum: electronegativities
Pauling electronegativity: 2.28 (Pauling units)
Allred Rochow electronegativity: 1.44 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Platinum: orbital properties
First ionisation energy: 864.40 kJ mol‑1
Second ionisation energy: 1791 kJ mol‑1
Third ionisation energy: 2800 kJ mol‑1
Platinum: abundances
Universe: 5 ppb by weight
Crustal rocks: 37 ppb by weight
Human: (no data) ppb by weight
Platinum: crystal structure
Platinum: biological data
Human abundance by weight: (no data) ppb by weight
Platinum has no biological role.
Platinum: uses
Platinum: reactions
Reactions of platinum as the element with air, water, halogens, acids, and bases where known.
Platinum: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of platinum where known.
Platinum: compound properties
Bond strengths; lattice energies of platinum halides, hydrides, oxides (where known); and reduction potentials where known.
Platinum: history
Platinum was discovered by Antonio de Ulloa in 1735 at South America. Origin of name: from the Spanish word "platina" meaning "silver".Platinum: isotopes
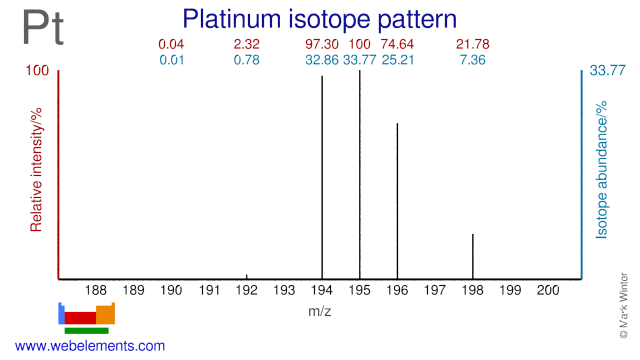
- Platinum isotopes are used in the following fields. Pt-196 is used in experiments to test nuclear models. Both Pt-194 and Pt-196 have been used in research into dipole strength and models. Pt-195 is used for the production of the radioisotope Pt-195m which is used for cancer diagnosis and therapy. Pt-198 is used for the production of the radioisotope for Au-199 which is used in cancer therapy. Pt-194 is also used for the production of the medical radioisotopes Hg-195m.
Platinum: isolation
Isolation: it would not normally be necessary to make a sample of platinum in the laboratory as the metal is available commercially. The industrial extraction of platinum is complex as the metal occurs in ores mixed with other metals such as palladium and gold. Sometimes extraction of the precious metals such as platinum and palladium is the main focus of a partiular industrial operation while in other cases it is a byproduct. The extraction is complex and only worthwhile since platinum is the basis of important catalysts in industry.
Preliminary treatment of the ore or base metal byproduct with aqua regia (a mixture of hydrochloric acid, HCl, and nitric acid, HNO3) gives a solution containing complexes of gold and palladium as well as H2PtCl6. The gold is removed from this solution as a precipitate by treatment with iron chloride (FeCl2). The platinum is precipitated out as impure (NH4)2PtCl6 on treatment with NH4Cl, leaving H2PdCl4 in solution. The (NH4)2PtCl6 is burned to leave an impure platinum sponge. This can be purified by redissolving in aqua regia, removal of rhodium and iridium impurities by treatment of the solution with sdoium bromate, and precipitation of pure (NH4)2PtCl6 by treatment with ammonium hydroxide, NH4OH. This yields platinum metal by burning.