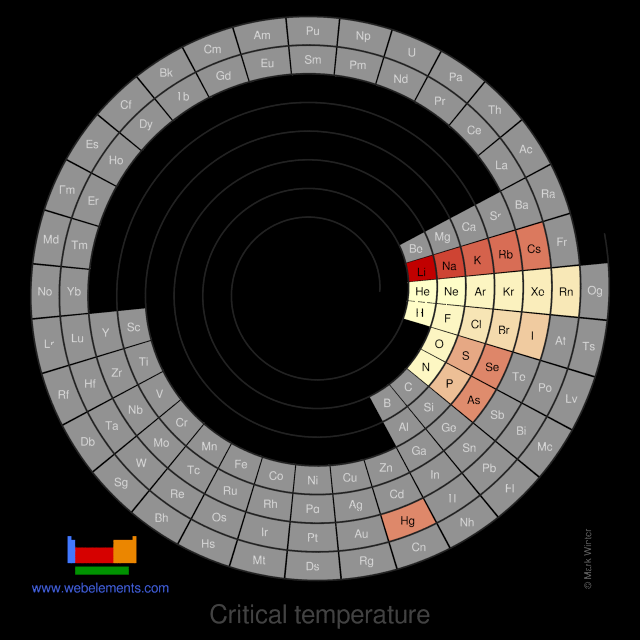
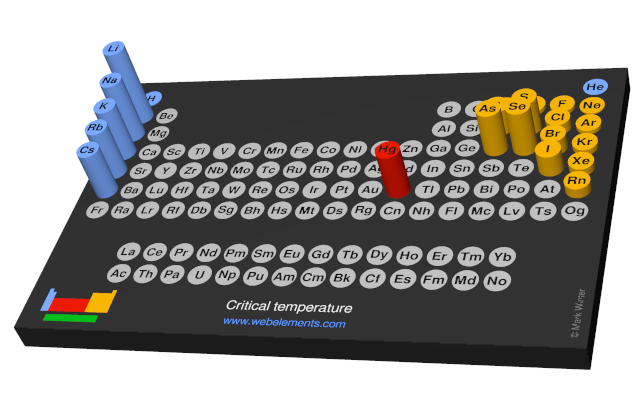
Critical temperature
The critical temperature is the temperature above which it is no longer possible to liquify the substance in question by increasing the pressure.



Units
K
Notes
Values given for the Group 1 elements (reference 1) are obtained by extrapolation.
Other temperature scales include the centigrade (Celsius) scale and the Fahrenheit scale. The size of one degree is the same on the Kelvin scale (K) as on the centigrade scale (°C). The zero point is different:
temperature (K) = temperature (°C) + 273.15
Thus, the melting point of water is = 0°C = 273.15 K and the boiling point of water is = 100°C = 373.15 K
On the Fahrenheit scale (°F), the melting point of water = 32°F while the boiling point = 212°F.
Therefore the degree size is different on the Fahrenheit scale with 180 Fahrenheit degrees = 100 centigrade degrees. Conversion between centigrade and Fahrenheit is achieved using the following relationship:
temperature (°C) = [temperature (°F) -32] * 5/9
Literature sources
- D. Ambrose in Chemical Rubber Company handbook of chemistry and physics, D.R. Lide (ed.) CRC Press, Boca Raton, Florida, USA, 77th edition, 1996.
- J.A. Dean (ed) in Lange's Handbook of Chemistry, McGraw-Hill, New York, USA, 14th edition, 1992.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||