Aluminium - 13Al: the essentials
- Name: aluminium
- Symbol: Al
- Atomic number: 13
- Relative atomic mass (Ar): 26.9815384 (3)
- Standard state: solid at 298 K
- Appearance: silvery
- Classification: Metallic
- Group in periodic table: 13
- Group name: (none)
- Period in periodic table: 3
- Block in periodic table: p
- Shell structure: 2.8.3
- CAS Registry: 7429-90-5
Aluminium atoms have 13 electrons and the shell structure is 2.8.3. The ground state electronic configuration of neutral aluminium is [Ne].3s2.3p1 and the term symbol of aluminium is 2P1/2.
Aluminium: description
Pure aluminium is a silvery-white metal with many desirable characteristics. It is light, nontoxic (as the metal), nonmagnetic and nonsparking. It is somewhat decorative. It is easily formed, machined, and cast. Pure aluminium is soft and lacks strength, but alloys with small amounts of copper, magnesium, silicon, manganese, and other elements have very useful properties. Aluminium is an abundant element in the earth's crust, but it is not found free in nature. The Bayer process is used to refine aluminium from bauxite, an aluminium ore.

Aluminium: physical properties
Density of solid: 2700 kg m-3
Molar volume: 10.00 cm3
Thermal conductivity: 235 W m‑1 K‑1
Aluminium: heat properties
Melting point: 933.47 [660.32 °C (1220.58 °F)] K
Boiling point: 2792 [2519 °C (4566 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Aluminium: atom sizes
Atomic radius (empirical): 125 pm
Molecular single bond covalent radius: 126 (coordination number 3) ppm
van der Waals radius: 225 ppm
Aluminium: electronegativities
Pauling electronegativity: 1.61 (Pauling units)
Allred Rochow electronegativity: 1.47 (Pauling units)
Mulliken-Jaffe electronegativity: 1.83 (sp2 orbital)
Aluminium: orbital properties
First ionisation energy: 577.54 kJ mol‑1
Second ionisation energy: 1816.68 kJ mol‑1
Third ionisation energy: 2744.78 kJ mol‑1
Aluminium: abundances
Universe: 50000 ppb by weight
Crustal rocks: 82000000 ppb by weight
Human: 900 ppb by weight
Aluminium: crystal structure
Aluminium: biological data
Human abundance by weight: 900 ppb by weight
Aluminium may be involved in the action of enzymes such a succinic dehydrogenase and δ-aminolevulinate dehydrase (involved in porphyrin synthesis. Aluminium compounds are toxic to most plants and somewhat toxic to mammals. Aluminium has been linked to Alzheimer's disease (senile dementia).
Aluminium: uses
Aluminium: reactions
Reactions of aluminium as the element with air, water, halogens, acids, and bases where known.
Aluminium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of aluminium where known.
Aluminium: compound properties
Bond strengths; lattice energies of aluminium halides, hydrides, oxides (where known); and reduction potentials where known.
Aluminium: history
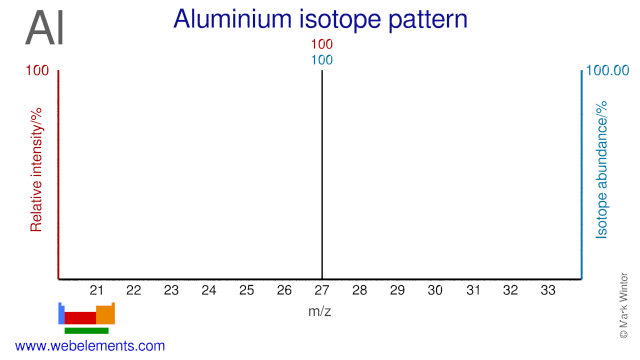
Aluminium was discovered by Hans Christian Oersted in 1825 at Denmark. Origin of name: from the Latin word "alumen" meaning "alum".Aluminium: isotopes
Aluminium: isolation
Isolation: aluminium would not mormally be made in the laboratory as it is so readily available commercially.
Aluminium is mined in huge scales as bauxite (typically Al2O3.2H2O). Bauxite contains Fe2O3, SiO2, and other impurities. In order to isolate pure aluminium, these impurities must be removed from the bauxite. This is done by the Bayer process. This involves treatment with sodium hydroxide (NaOH) solution, which results in a solution of sodium aluminate and sodium silicate. The iron remains behind as a solid. When CO2 is blown through the resulting solution, the sodium silicate stays in solution while the aluminium is precipitated out as aluminium hydroxide. The hydroxide can be filtered off, washed, and heated to form pure alumina, Al2O3.
The next stage is formation of pure aluminium. This is obtained from the pure Al2O3 by an electrolytic method. Electrolysis is necessary as aluminium is so electropositive. It seems these days that electrolysis of the hot oxide in a carbon lined steel cell acting as the cathode with carbon anodes is most common.