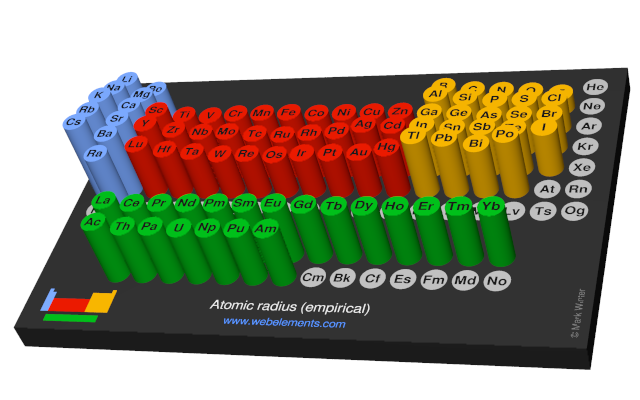
Atomic radius (empirical)
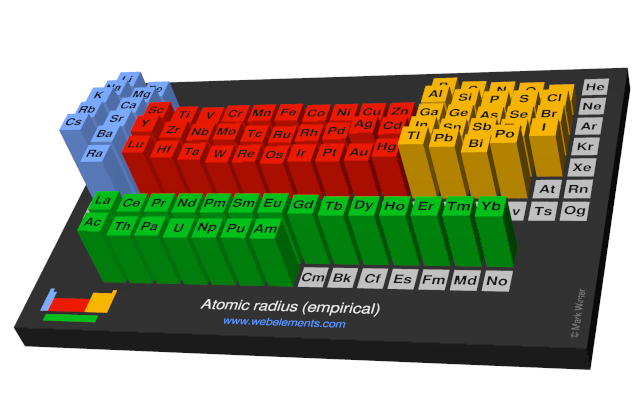
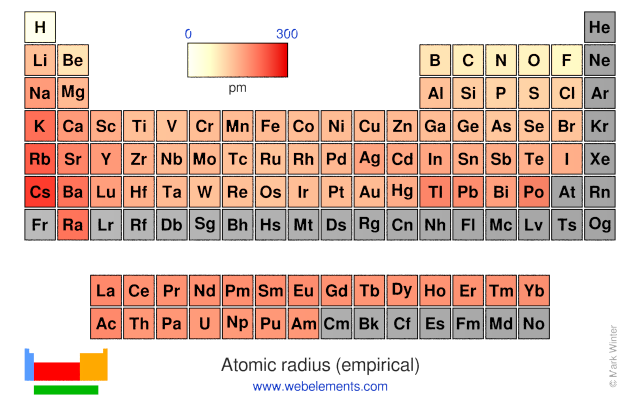
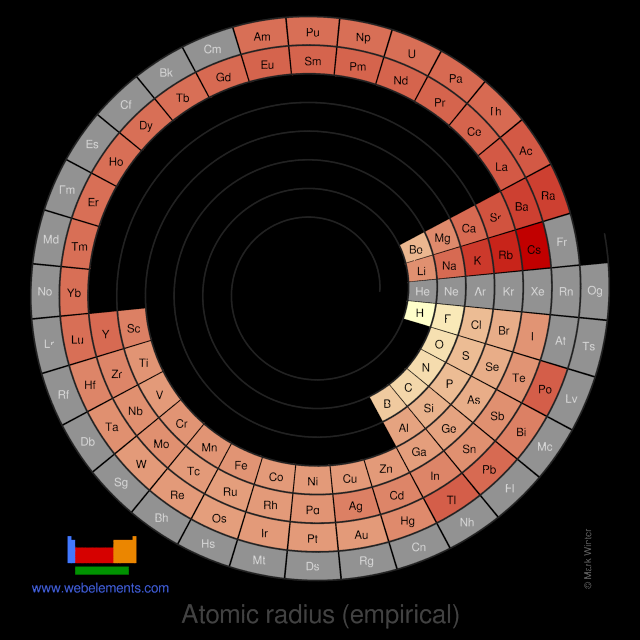
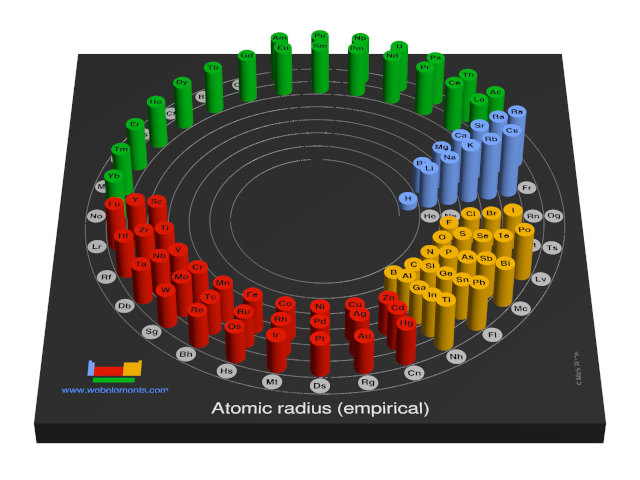
These values derived by J.C. Slater are an empirical set of atomic radii derived by the careful comparison of bond lengths in over 1200 bond types in ionic, metallic, and covalent crystals and molecules (reference 1).
The idea is that for a bond A-B, the atomic radius of A added to the atomic radius of B will give a reasonable estimate for the A-B separation in whatever environment. A single set of radii is very useful for most purposes, however, for very accurate work adjustments would have to be made to the values quoted to reflect the specific environment of the element (such as coordination number). Values are given to an accuracy of about 5 pm.
Units
pm
Notes
None
Select from the following links to see visual periodicity representations for atomic radii, covalent radii, and van der Waals radii. Ionic radii are also available.
- Atomic radii
- Atomic radii (empirical)
- Atomic radii (absolute)
- Atomic radii (density cutoff)
- Covalent radii
- Covalent radii revisited (2008 values)
- Covalent radii (molecular single bond)
- Covalent radii (molecular double bond)
- Covalent radii (molecular triple bond)
- Van der Waals radius
Literature sources
- J.C. Slater, J. Chem. Phys. 1964, 39, 3199.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||