Molybdenum - 42Mo: the essentials
- Name: molybdenum
- Symbol: Mo
- Atomic number: 42
- Relative atomic mass (Ar): 95.95 (1) g [see note g]
- Standard state: solid at 298 K
- Appearance: grey metallic
- Classification: Metallic
- Group in periodic table: 6
- Group name: (none)
- Period in periodic table: 5
- Block in periodic table: d
- Shell structure: 2.8.18.13.1
- CAS Registry: 7439-98-7
Molybdenum atoms have 42 electrons and the shell structure is 2.8.18.13.1. The ground state electronic configuration of neutral molybdenum is [Kr].4d5.5s1 and the term symbol of molybdenum is 7S3.
Molybdenum: description
Molybdenum is a silvery-white, hard, transition metal. Scheele discovered it in 1778. It was often confused with graphite and lead ore. Molybdenum is used in alloys, electrodes and catalysts. The World War 2 German artillery piece called "Big Bertha" contains molybdenum as an essential component of its steel.

Molybdenum rods.
Molybdenum: physical properties
Density of solid: 10280 kg m-3
Molar volume: 9.38 cm3
Thermal conductivity: 139 W m‑1 K‑1
Molybdenum: heat properties
Melting point: 2896 [2623 °C (4753 °F)] K
Boiling point: 4912 [4639 °C (8382 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Molybdenum: atom sizes
Atomic radius (empirical): 145 pm
Molecular single bond covalent radius: 138 (coordination number 6) ppm
van der Waals radius: 245 ppm
Molybdenum: electronegativities
Pauling electronegativity: 2.16 (Pauling units)
Allred Rochow electronegativity: 1.30 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Molybdenum: orbital properties
First ionisation energy: 684.32 kJ mol‑1
Second ionisation energy: 1559 kJ mol‑1
Third ionisation energy: 2618 kJ mol‑1
Molybdenum: abundances
Universe: 5 ppb by weight
Crustal rocks: 1100 ppb by weight
Human: 100 ppb by weight
Molybdenum: crystal structure
Molybdenum: biological data
Human abundance by weight: 100 ppb by weight
Molybdenum is a necessary element, apparently for all species. Only very small amounts are required. Molybdenum plays a role in nitrogen fixation, (a process by which the normally unreactive nitrogen gas is turned into other compounds) enzymes, and nitrate reduction enzymes.
Molybdenum: uses
Molybdenum: reactions
Reactions of molybdenum as the element with air, water, halogens, acids, and bases where known.
Molybdenum: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of molybdenum where known.
Molybdenum: compound properties
Bond strengths; lattice energies of molybdenum halides, hydrides, oxides (where known); and reduction potentials where known.
Molybdenum: history
Molybdenum was discovered by Carl William Scheele in 1781 at Sweden. Origin of name: from the Greek word "molybdos" meaning "lead".Molybdenum: isotopes
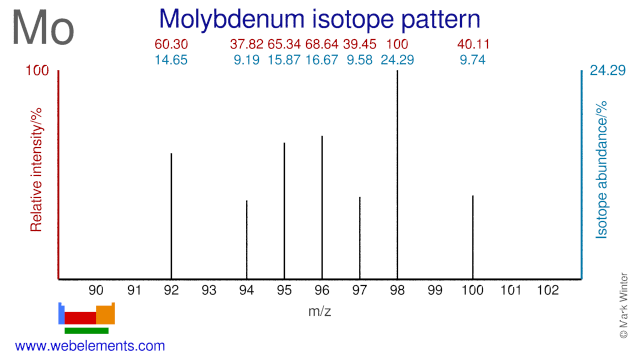
The Molybdenum isotope Mo-95 is used for the production of the medical radioisotope Ru-97. Mo-96 is used for the production of the radioisotopes Tc-96 and Tc-95m, both of which have a medical application. Most Mo isotopes are also used in nutrition studies in humans. Depleted Mo-95 has been suggested for use in UMo fuel elements for materials test (high flux) reactors.
Molybdenum: isolation
Isolation: it is not normally necessary to make samples of molybdenum metal in the laboratory since it is readily available commercially. Industrially, its extraction is sometimes linked to copper production. The normal process is for the sulphide MoS2 to be "roasted" to form the oxide MoO3. This is often used directly in the steel industry.
Pure samples of the metal are available by first dissolving the oxide in ammonium hydroxide to make ammonium molybdate, (NH4)2[MO4], and thenreduction of the molybdate with hydrogen gas to form the metal.