Gold - 79Au: the essentials
- Name: gold
- Symbol: Au
- Atomic number: 79
- Relative atomic mass (Ar): 196.966570 (4)
- Standard state: solid at 298 K
- Appearance: gold
- Classification: Metallic
- Group in periodic table: 11
- Group name: Coinage metal
- Period in periodic table: 6
- Block in periodic table: d
- Shell structure: 2.8.18.32.18.1
- CAS Registry: 7440-57-5
Gold atoms have 79 electrons and the shell structure is 2.8.18.32.18.1. The ground state electronic configuration of neutral gold is [Xe].4f14.5d10.6s1 and the term symbol of gold is 2S1/2.
Gold: description
Most metals are metallic grey or silvery white whereas gold is characteristically a metallic yellow colour, in other words gold-coloured. Caesium is also gold coloured. The gold colour seems related to relativistic effects of the outermost gold orbitals.
Small amounts of other metals alloyed with gold change the colour as well as mechanical properties such as hardness. White gold for jewellery is formed by mixing palladium, silver, or nickel with gold, although the result is green gold with certain proportions of silver. White gold is commonly used for wedding rings in the USA. Addition of some copper gives "rose gold", a soft pink colour. Remarkably other colours such as purple (a gold:aluminium alloy), blue (a gold:indium alloy) and even black (a gold:cobalt alloy) may be formed.
Gold is usually alloyed in jewellery to give it more strength, and the term carat describes the amount of gold present (24 carats is pure gold). It is estimated that all the gold in the world, so far refined, could be placed in a single cube 60 ft. on a side. It is metallic, with a yellow colour when in a mass, but when finely divided it may be black, ruby, or purple.
It is the most malleable and ductile metal; 1 ounce (28 g) of gold can be beaten out to 300 square feet. It is a soft metal and is usually alloyed to give it more strength. It is a good conductor of heat and electricity, and is unaffected by air and most reagents.
Gold is readily available commercially and its price changes day by day and is one of the most widely tracked commercial prices.
The most common gold compounds are auric chloride (AuCl3) and chlorauric acid (HAuCl4). A mixture of one part nitric acid with three of hydrochloric acid is called aqua regia (because it dissolved gold, the King of Metals). It is unaffected by air and most reagents. It is found free in nature and associated with quartz, pyrite and other minerals. Two thirds of the world's supply comes from South Africa, and 2/3 of USA production is from South Dakota and Nevada. Gold is found in sea water, but no effective economic process has been designed (yet) to extract it from this source.

Gold Assay
It is critical from the public's perspective that there is confidence in the claimed purity of any particular item made from precious metals. This confidence is provided by an 'assay' (test and assess) of the precious metal content of that item. It is impossible to tell the precious metal content of any item simply by looking at it. Precious metals such as old, silver, and platinum are too soft to use alone for making jewellery, cutlery and other goods. Quite properly they must be alloyed with base metals (which happen to be cheap) for manufacturing. The assay protects the consumer by ensuring sure that not too much base metal was used. It also safeguards responsible manufacturers by providing an independent assessment of quality and content that in which the public has confidence.
There has been an "Assay Office" at Sheffield in England since 1773 when local silversmiths won the right from Parliament to assay silver in Sheffield. The 1773 Act of Parliament appointed 30 local men as 'Guardians of the Standard of Wrought Plate in the Town of Sheffield' to supervise the work of the Office. In 1773 Sheffield already had an established tradition of fine silverware production and the number of Guardians who were also silversmiths was restricted to just ten to ensure that the Assay Office offered an independent and impartial service. This safeguard was to ensure the Office was run for the benefit of the consumer as well as the manufacturer. Once a piece was assayed, it was marked using a "hallmark", making the hallmark perhaps the oldest mark of consumer protection. The first UK Assay Office was and is based at Goldsmiths' Hall in London. It founded around 1300, and is from where the term "hallmarking" originates, meaning "marked in Goldsmiths' Hall".
While there are assay offices in the USA, there is no hallmarking scheme.
Cartoon by Nick D Kim ([Science and Ink], used by permission).
Gold: physical properties
Density of solid: 19300 kg m-3
Molar volume: 10.21 cm3
Thermal conductivity: 320 W m‑1 K‑1
Gold: heat properties
Melting point: 1337.33 [1064.18 °C (1947.52 °F)] K
Boiling point: 3129 [2856 °C (5173 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Gold: atom sizes
Atomic radius (empirical): 135 pm
Molecular single bond covalent radius: 124 (coordination number 2) ppm
van der Waals radius: 245 ppm
Gold: electronegativities
Pauling electronegativity: 2.54 (Pauling units)
Allred Rochow electronegativity: 1.42 (Pauling units)
Mulliken-Jaffe electronegativity: 1.87 (s orbital)
Gold: orbital properties
First ionisation energy: 890.13 kJ mol‑1
Second ionisation energy: 1949.3 kJ mol‑1
Third ionisation energy: 2890 kJ mol‑1
Gold: abundances
Universe: 0.6 ppb by weight
Crustal rocks: 3.1 ppb by weight
Human: 100 ppb by weight
Gold: crystal structure
Gold: biological data
Human abundance by weight: 100 ppb by weight
Gold is not a necessary trace element for living things and neither are any of the other third row d-block elements. In part this may be because there is little gold in the biosphere with which living things might experiment and also because there are few ways for living things to convert gold into a suitable soluble form. There do seem to be a few plants that accumulate gold, perhaps because they are associated with microorganisms containing particular amino acids that complex gold.
Given the fascination of people for gold it is not surprising that people experimented with gold for medical treatments. In the 8th century alchemists attempted to prepare elixirs from metallic gold. These were supposed to cure all diseases as well as conferring eternal youth. In the 13th century gold was disolved in aqua regia and the resultant mixture mixed with oil of rosemary or other "essential oils" to form aurum potabile. This was said to cure leprosy. Following this gold treatments were often often used for any number of conditions but with little evidence that they actually worked. Things started to get a little more interesting in the 1890s when it was found that the gold cyanide salt K[Au(CN)2] killed the microorganism responsible for tuberculosis. This was used to treat tuberculosis but, perhaps unsurprisingly, with considerable toxicity problems.
After the first world war gold thiol drugs were developed. These are Au(I) complexes such as sodium aurothiosulphate ("Sanocrysin"), sodium aurothiomalate ("Myocrisin"), and aurothioglucose ("Solganal-B-oleosum"). These are less toxic than K[Au(CN)2] but eventually fell out of favour. However sodium aurothiosulphate and aurothioglucose ("Solganal-B-oleosum") are in use today for the treatment of rheumatoid arthritis following successful drug trials. Some patients do suffer side effects.
More recently gold phosphine complexes of the type [AuX(PR3)] (X = halide, R = alkyl) were tested for anti-inflammatory effects. They possess the advantage that they may be taken orally whereas earlier drugs such as sodium aurothiomalate must be injected. The gold phosphine drugs seem less toxic to the kidneys and the best therapeutic effect is observed for [AuCl(PEt3)].
Auranofin (brand name Ridaura) is an organogold compound classified by the World Health Organization as an antirheumatic agent. IUPAC Name: gold(1+); 3,4,5-triacetyloxy-6-(acetyloxymethyl)oxane-2-thiolate; triethylphosphanium Canonical SMILES: CC[PH+](CC)CC.CC(=O)OCC1C(C(C(C(O1)[S-])OC(=O)C)OC(=O)C)OC(=O)C.[Au+] InChI: InChI=1S/C14H20O9S.C6H15P.Au/c1-6(15)19-5-10-11(20-7(2)16)12(21-8(3)17) 13(14(24)23-10)22-9(4)18;1-4-7(5-2)6-3;/h10-14,24H,5H2,1-4H3;4-6H2, 1-3H3;/q;;+1
Other drugs that perhaps have anti-inflammatory effects include Au{(SCH(CH2CO2H)(CO2H)}(PR3)] (R = alkyl, alkoxy, phenyl).
The gold isotope 198Au is used for treating cancer and other conditions.
Further reading:
Peter J. Sadler, "The biological chemistry of gold", Gold Bulletin, 1976, 9, 110-118.
Sabine L Best and Peter J. Sadler, "Gold Drugs: Mechanism of Action and Toxicity", Gold Bulletin, 1996, 29, 87-93.
Gold: uses
Gold: reactions
Reactions of gold as the element with air, water, halogens, acids, and bases where known.
Gold: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of gold where known.
Gold: compound properties
Bond strengths; lattice energies of gold halides, hydrides, oxides (where known); and reduction potentials where known.
Gold: history
Gold was discovered by known since ancient times in unknown at not known. Origin of name: from the Anglo-Saxon word "gold" (the origin of the symbol Au is the Latin word "aurum" meaning "gold").Gold: isotopes
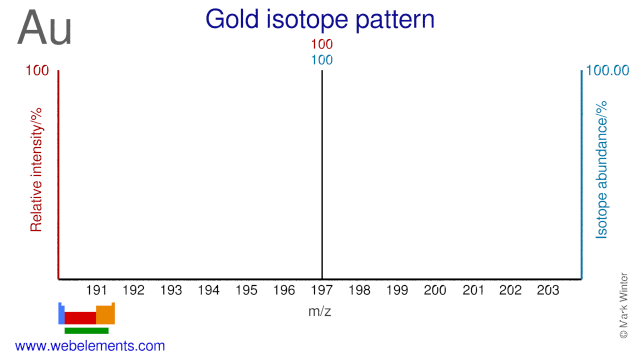
Gold is monoisotopic consisting completely of 197Au. However there are a number of radioisotopes, some of which are listed.
The gold isotope 198Au is used for treating cancer and other conditions. In the form of a gold colloid, 198Au has a diagnostic use for liver imaging and a therapeutic use in treatment of widespread abdominal carcinomatosis with ascites; carcinomatosis of pleura with effusion; lymphomas; interstitially in metastatic tumour. While there is a certain risk from the β-decay of 198Au the calculation is that the benefits outweight the risks.
Gold: isolation
Isolation: it would not normally be necessary to make gold in the laboratory as it is readily available commercially. The most romantic way to extract gold is by panning it out from a stream in some pleasant valley but most such sources are now depleted. Panning relies upon the density of gold (which is very high) being much greater than that of the sand and other particulates. It therefore settles to the bottom of the pan. The amount of gold recoverable in this way is declining.
One suggestion regarding the golden fleece in the Jason and the Argonauts story (Greek mythology) is that the golden fleece is a consequence of gold mining. There are suggestions that, perhaps 1500 years ago sheep fleeces were stretched out over wooden frames and be submerged in streams. Gold particles swept down from from upstream deposits would then become embedded in them. The fleeces were then dried in trees before shaking or combing the gold out. Similarly, sheep fleeces may have been used on washing tables at alluvial gold mines with much the same effect. Perhaps such methods predated panning of gold from river sands.
Today, more often than not, gold is extracted from ores. These ores often contain relatively little gold. Some of these processes cause environmental concern. Much gold is recovered from ores that are low in gold concentration using a cyanide extraction process. Cyanide extraction was first used around 1887, when the MacArthur-Forrest Process was developed in Glasgow by John Stewart MacArthur. Many worry about the envoronmental effects of the cyanide extraction process and the risks of using cyanide on a large scale. There are three main steps.
The first step is leaching - the ore is crushed to a powder so as to expose the small gold particles. and mixed with water. The resulting mixture of powdered ore and water (the slury) is then reacted with cyanide in the presence of oxygen.
4Au(s) + 8CN-(aq) + 2H2O(l) + O2(g) → 4[Au(CN)2]-(aq) + 4OH-(aq)
The result is that electrons from oxygen are used to convert the gold metal into a Au(I) complex, [Au(CN)2]-. The acidity of the process must be slightly alkaline (pH 10.1 for instance) to minimise the release of highly toxic hydrogen cyanide while optimising the leaching rate.
The next stage is concentration. Once in solution the gold must be converted back to gold metal. One way to do this is by adsorption of the gold onto activated carbon. Most of the impurities are left behind in the solution. This would appear to cause some anion exchange of [Au(CN)2]- with anions associated with the carbon, the precipitation of insoluble AuCN, and the formation of some metallic gold within the carbon pore structure.
The final step is recovery and refining. Gold is stripped from the carbon by mixing it with NaCN and NaOH at 1108C forming a new solution of [Au(CN)2]-. This solution is now fairly pure as the activated carbon process removes many of the impurities. The gold is then converted back to elemental gold in the following electrolysis reactions known as "electrowinning":
At the anode: 4OH- → O2 + 2H2O +4e-
At the cathode: e- + [Au(CN)2]- → Au + 2CN-
Overall: 4OH- + 4[Au(CN)2]- → 4Au + 8CN- + O2 + 2H2O
The gold is "won" onto stainless steel electrodes or precipitated out as a fine black mud. The mud is then smelted and poured into moulds to make gold ingots.
As an laternative to this, zinc powder is added to the solution or reasonably pure of [Au(CN)2]-. This results in a metal displacement reaction:
2[Au(CN)2]-(aq) + Zn(s) -> [Zn(CN)4]2-(aq) + 2Au(s)
As before a fine black 'mud' of gold and residual zinc precipitates from the solution, which is then smelted.
Extraction of gold from seawater
There is some gold in seawater, but the concentration of dissolved gold is very low, perhaps 10 ng l-1. The most determined attempt to recover gold from sea water was undertaken by Fritz Haber, [F. Haber, Z. Angew. Chem. 1927, 40, 303.], who researched the matter extensively after the First World War. He wanted to find a way to pay Germany's war reparation debts. He developed a method involving gold reduction to the metal by sodium polysulfide and removal using sulphur-coated sand filters. Four expeditions were made on ships equipped with the extraction technology but with disappointing results. Following this Haber estimated the gold concentration in sea water to be 4 ng l-1, just one-thousandth of the amount which he had expected.
With the current estimate for the concentration of gold in sea water as 10 ng-1 and the total volume of the oceans at 1.37 x 109 km3, then the total quantity of gold dissolved in sea water is calculated to be 13.7 million tons. A lot of gold, but not extractable on a commercial basis as yet.