Tantalum - 73Ta: the essentials
- Name: tantalum
- Symbol: Ta
- Atomic number: 73
- Relative atomic mass (Ar): 180.94788 (2)
- Standard state: solid at 298 K
- Appearance: grey blue
- Classification: Metallic
- Group in periodic table: 5
- Group name: (none)
- Period in periodic table: 6
- Block in periodic table: d
- Shell structure: 2.8.18.32.11.2
- CAS Registry: 7440-25-7
Tantalum atoms have 73 electrons and the shell structure is 2.8.18.32.11.2. The ground state electronic configuration of neutral tantalum is [Xe].4f14.5d3.6s2 and the term symbol of tantalum is 4F3/2.
Tantalum: description
Tantalum is a greyish silver, heavy, and very hard metal. When pure, it is ductile and can be drawn into fine wire, which can be used as a filament for evaporating metals such as aluminium. Tantalum is almost completely immune to chemical attack at temperatures below 150°C, and is attacked only by hydrofluoric acid, acidic solutions containing the fluoride ion, and free sulphur trioxide. The element has a melting point exceeded only by tungsten and rhenium.

Small and large samples of tantalum wire like this, as well as foil, sheet, insulated wire, mesh and rod, can be purchased via their web catalogue from Advent Research Materials via their web catalogue.
Tantalum: physical properties
Density of solid: 16650 kg m-3
Molar volume: 10.85 cm3
Thermal conductivity: 57 W m‑1 K‑1
Tantalum: heat properties
Melting point: 3290 [3017 °C (5463 °F)] K
Boiling point: 5731 [5458 °C (9856 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Tantalum: atom sizes
Atomic radius (empirical): 145 pm
Molecular single bond covalent radius: 146 (coordination number 5) ppm
van der Waals radius: 257 ppm
Tantalum: electronegativities
Pauling electronegativity: 1.5 (Pauling units)
Allred Rochow electronegativity: 1.33 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Tantalum: orbital properties
First ionisation energy: 728.42 kJ mol‑1
Second ionisation energy: 1560 kJ mol‑1
Third ionisation energy: 2230 kJ mol‑1
Tantalum: abundances
Universe: 0.08 ppb by weight
Crustal rocks: 1700 ppb by weight
Human: (no data) ppb by weight
Tantalum: crystal structure
Tantalum: biological data
Human abundance by weight: (no data) ppb by weight
Tantalum has no biological role. Possibly some tantalum compounds cause tumours.
Tantalum: uses
Tantalum: reactions
Reactions of tantalum as the element with air, water, halogens, acids, and bases where known.
Tantalum: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of tantalum where known.
Tantalum: compound properties
Bond strengths; lattice energies of tantalum halides, hydrides, oxides (where known); and reduction potentials where known.
Tantalum: history
Tantalum was discovered by Anders Ekeberg in 1802 at Sweden. Origin of name: from the Greek word "Tantalos" meaning "father of Niobe" (Greek mythology, (tantalum is closely related to niobium in the periodic table).Tantalum: isotopes
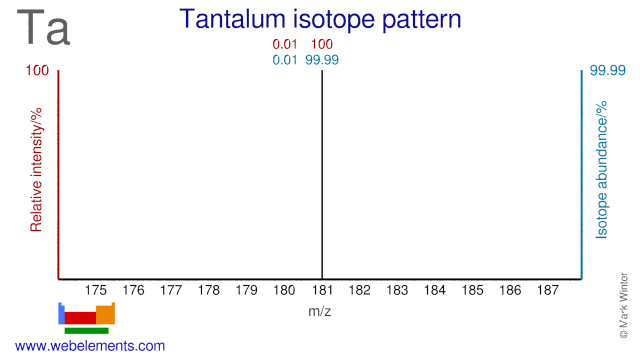
Tantalum has only two isotopes and one of them, Ta-180, has one of the lowest natural abundances of all naturally occurring isotopes (0.012%). Ta-180 has only been produced in minute quantities and is very expensive. Ta-181 can be used for the production of W-178 which decays to Ta-178. Ta-178 emits low energy gamma rays which can be used for imaging purposes.
Tantalum: isolation
Isolation: isolation of tantalum appears to be complicated. Tantalum minerals usually contain both niobium and tantalum. Since they are so similar chemically, it is difficult to separate them. Tantalum can be extracted from the ores by first fusing the ore with alkali, and then extracting the resultant mixture into hydrofluoric acid, HF. Current methodology involves the separation of tantalum from these acid solutions using a liquid-liquid extraction technique. In this process tantalum salts are extracted into the ketone MIBK (methyl isobutyl ketone, 4-methyl pentan-2-one). The niobium remains in the HF solution.
After conversion to the oxide, metallic tantalum can be made by reduction with sodium or carbon. Electrolysis of molten fluorides is also used.