Boron - 5B: the essentials
- Name: boron
- Symbol: B
- Atomic number: 5
- Relative atomic mass (Ar): 10.81 ±0.02. Range: [10.806, 10.821] m [see notes g m r]
- Standard state: solid at 298 K
- Appearance: black
- Classification: Semi-metallic
- Group in periodic table: 13
- Group name: (none)
- Period in periodic table: 2
- Block in periodic table: p
- Shell structure: 2.3
- CAS Registry: 7440-42-8
Boron atoms have 5 electrons and the shell structure is 2.3. The ground state electronic configuration of neutral boron is [He].2s2.2p1 and the term symbol of boron is 2P1/2.
Boron: description
Boron is a Group 13 element that has properties which are borderline between metals and non-metals (semimetallic). It is a semiconductor rather than a metallic conductor. Chemically it is closer to silicon than to aluminium, gallium, indium, and thallium.
Crystalline boron is inert chemically and is resistant to attack by boiling HF or HCl. When finely divided it is attacked slowly by hot concentrated nitric acid.

Image adapted with permission from Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Boron: physical properties
Density of solid: 2460 kg m-3
Molar volume: 4.39 cm3
Thermal conductivity: 27 W m‑1 K‑1
Boron: heat properties
Melting point: 2349 [2076 °C (3769 °F)] K
Boiling point: 4200 [3927 °C (7101 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Boron: atom sizes
Atomic radius (empirical): 85 pm
Molecular single bond covalent radius: 85 (coordination number 3) ppm
van der Waals radius: 191 ppm
Boron: electronegativities
Pauling electronegativity: 2.04 (Pauling units)
Allred Rochow electronegativity: 2.01 (Pauling units)
Mulliken-Jaffe electronegativity: 2.04 (sp2 orbital)
Boron: orbital properties
First ionisation energy: 800.64 kJ mol‑1
Second ionisation energy: 2427.07 kJ mol‑1
Third ionisation energy: 3659.74 kJ mol‑1
Boron: abundances
Universe: 1 ppb by weight
Crustal rocks: 8700 ppb by weight
Human: 700 ppb by weight
Boron: crystal structure
Boron: biological data
Human abundance by weight: 700 ppb by weight
Boron is probably not required in the diet of humans but it might be a necessary "ultratrace" element. Boron is required by green algae and higher plants.
Boron: uses
Boron: reactions
Reactions of boron as the element with air, water, halogens, acids, and bases where known.
Boron: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of boron where known.
Boron: compound properties
Bond strengths; lattice energies of boron halides, hydrides, oxides (where known); and reduction potentials where known.
Boron: history
Boron was discovered by Sir Humphrey Davy, Joseph-Louis Gay-Lussac, L.J. Thénard in 1808 at England and France. Origin of name: from the Arabic word "buraq" and the Persian word "burah" .Boron: isotopes
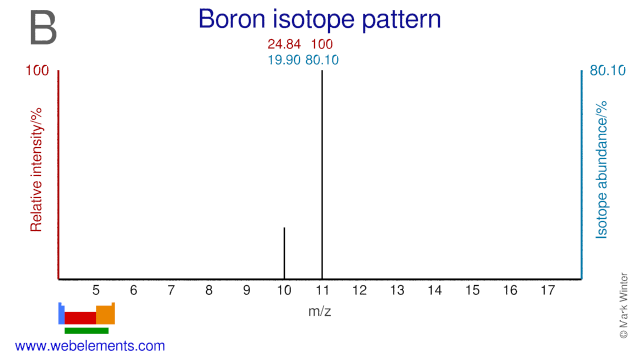
Both isotopes of Boron, B-10 and B-11, are used extensively in the nuclear industry. B-10 is used in the form of boric acid as a chemical shim in pressurized water reactors while in the form of sodium pentaborate it is used for standby liquid control systems in boiling water reactors. B-11 can be used as a neutron reflector. Outside the nuclear industry both isotopes are used as food label to study boron metabolism. B-10 is also used in so-called boron neutron capture therapy (BNCT). Both B-10 and B-11 can be used for the production of two radioisotopes: C-11 and N-13.
Boron: isolation
Isolation: it is not normally necessary to make boron in the laboratory and it would normally be purchased as it is available commercially. The most common sources of boron are tourmaline, borax [Na2B4O5(OH)4.8H2O], and kernite [Na2B4O5(OH)4.2H2O]. It is difficult to obtain pure. It can be made through the magnesium reduction of the oxide, B2O3. The oxide is made by melting boric acid, B(OH)3, which in turn is obtained from borax.
B2O3 + 3Mg → 2B + 3MgO
Samm amounts of high purity boron are available through the thermal decomposition of compounds such as BBr3 with hydrogen gas using a heated tantalum wire. Results are better with hot wires at tmeperatures over 1000°C.