Palladium - 46Pd: the essentials
- Name: palladium
- Symbol: Pd
- Atomic number: 46
- Relative atomic mass (Ar): 106.42 (1) g [see note g]
- Standard state: solid at 298 K
- Appearance: silvery white metallic
- Classification: Metallic
- Group in periodic table: 10
- Group name: Precious metal or Platinum group metal
- Period in periodic table: 5
- Block in periodic table: d
- Shell structure: 2.8.18.18.0
- CAS Registry: 7440-05-3
Palladium atoms have 46 electrons and the shell structure is 2.8.18.18.0. The ground state electronic configuration of neutral palladium is [Kr].4d10 and the term symbol of palladium is 1S0.
Palladium: description
Palladium is a steel-white metal, does not tarnish in air, and is the least dense and lowest melting of the platinum group metals. When annealed, it is soft and ductile. Cold working increases its strength and hardness. It is used in some watch springs.
Ruthenium, rhodium, palladium, osmium, iridium, and platinum together make up a group of elements referred to as the platinum group metals (PGM).
At room temperatures the metal has the unusual property of absorbing up to 900 times its own volume of hydrogen. Hydrogen readily diffuses through heated palladium and this provides a means of purifying the gas.

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.
Palladium: physical properties
Density of solid: 12023 kg m-3
Molar volume: 8.56 cm3
Thermal conductivity: 72 W m‑1 K‑1
Palladium: heat properties
Melting point: 1828.05 [1554.9 °C (2830.82 °F)] K
Boiling point: 3236 [2963 °C (5365 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Palladium: atom sizes
Atomic radius (empirical): 140 pm
Molecular single bond covalent radius: 120 (coordination number 3) ppm
van der Waals radius: 215 ppm
Palladium: electronegativities
Pauling electronegativity: 2.20 (Pauling units)
Allred Rochow electronegativity: 1.35 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Palladium: orbital properties
First ionisation energy: 804.38 kJ mol‑1
Second ionisation energy: 1875 kJ mol‑1
Third ionisation energy: 3177 kJ mol‑1
Palladium: abundances
Universe: 2 ppb by weight
Crustal rocks: 6.3 ppb by weight
Human: (no data) ppb by weight
Palladium: crystal structure
Palladium: biological data
Human abundance by weight: (no data) ppb by weight
Palladium has no biological role. Palladium chloride was formerly prescribed as a treatment for tuberculosis at the rate of 0.065 g per day (approximately 1 mg kg-1) without too many bad side effects.
Palladium: uses
Palladium: reactions
Reactions of palladium as the element with air, water, halogens, acids, and bases where known.
Palladium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of palladium where known.
Palladium: compound properties
Bond strengths; lattice energies of palladium halides, hydrides, oxides (where known); and reduction potentials where known.
Palladium: history
Palladium was discovered by William Hyde Wollaston in 1803 at England. Origin of name: named after the asteroid "Pallas" which was discovered at about the same time and from the Greek name "Pallas", goddess of wisdom.Palladium: isotopes
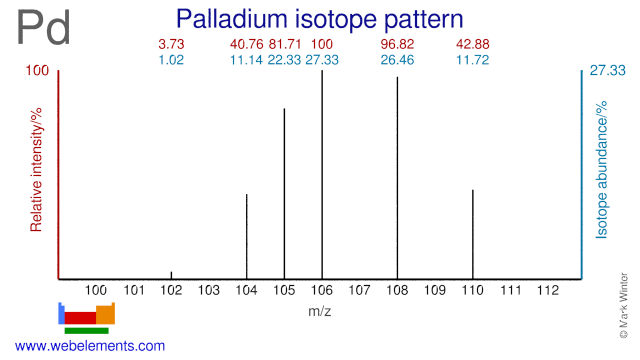
Pd-104 is used for the production of radioactive Pd-103 seeds which are used to fight prostate cancer. Pd-103 can also be made from Pd-102. Other Pd isotopes such as Pd-110 and Pd-108 have been used in physical experiments such as research into the decay of Nd-137 and nuclear fusion phenomena. Pd-108 can also be used for the production of radioactive Pd-109 which is used for cancer therapy.
Palladium: isolation
Isolation: it would not normally be necessary to make a sample of palladium in the laboratory as the metal is available commercially. The industrial extraction of palladium is complex as the metal occurs in ores mixed with other metals such as platinum. Sometimes extraction of the precious metals such as platinum and palladium is the main focus of a partiular industrial operation while in other cases it is a byproduct. The extraction is complex and only worthwhile since palladium is the basis of important catalysts in industry.
Preliminary treatment of the ore or base metal byproduct with aqua regia (a mixture of hydrochloric acid, HCl, and nitric acid, HNO3) gives a solution containing complexes of gold and platinum as well as H2PdCl4. The gold is removed from this solution as a precipitate by treatment with iron chloride (FeCl2). The platinum is precipitated out as (NH4)2PtCl6 on treatment with NH4Cl, leaving H2PdCl4 in solution. The palladium is precipitated out by treatment with ammonium hydroxide, NH4OH, and HCl as the complex PdCl2(NH3)2. This yields palladium metal by burning.