Gallium - 31Ga: the essentials
- Name: gallium
- Symbol: Ga
- Atomic number: 31
- Relative atomic mass (Ar): 69.723 (1)
- Standard state: solid at 298 K (but melts only slightly above this temperature)
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table: 13
- Group name: (none)
- Period in periodic table: 4
- Block in periodic table: p
- Shell structure: 2.8.18.3
- CAS Registry: 7440-55-3
Gallium atoms have 31 electrons and the shell structure is 2.8.18.3. The ground state electronic configuration of neutral gallium is [Ar].3d10.4s2.4p1 and the term symbol of gallium is 2P1/2.
Gallium: description
Gallium is the only metal, except for mercury, caesium, and rubidium, which can be liquid near room temperatures; this makes possible its use in high-temperature thermometers. It has one of the longest liquid ranges of any metal and has a low vapour pressure even at high temperatures.
Ultra-pure gallium has a beautiful, silvery appearance, and the solid metal exhibits a conchoidal fracture similar to glass. The metal expands on solidifying; therefore, it should not be stored in glass or metal containers, as they may break as the metal solidifies.
High-purity gallium is attacked only slowly by mineral acids. Gallium arsenide is capable of converting electricity directly into coherent light and gallium arsenide is a key component of LEDs (light emitting diodes). In the 1990s gallium nitride (GaN) was discovered to emit blue light in light-emitting diodes (LEDs). As red and green LEDs were already known, this meant that red green, and blue LEDS could be used in full-colour LED displays while white LEDs and blue laser devices became possible as well.

Image adapted with permission from Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Gallium: physical properties
Density of solid: 5904 kg m-3
Molar volume: 11.80 cm3
Thermal conductivity: 29 W m‑1 K‑1
Gallium: heat properties
Melting point: 302.91 [29.76 °C (85.57 °F)] K
Boiling point: 2477 [2204 °C (3999 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Gallium: atom sizes
Atomic radius (empirical): 130 pm
Molecular single bond covalent radius: 124 (coordination number 3) ppm
van der Waals radius: 232 ppm
Gallium: electronegativities
Pauling electronegativity: 1.81 (Pauling units)
Allred Rochow electronegativity: 1.82 (Pauling units)
Mulliken-Jaffe electronegativity: 2.01 (sp2 orbital)
Gallium: orbital properties
First ionisation energy: 578.84 kJ mol‑1
Second ionisation energy: 1979.41 kJ mol‑1
Third ionisation energy: 2964.58 kJ mol‑1
Gallium: abundances
Universe: 10 ppb by weight
Crustal rocks: 19000 ppb by weight
Human: (no data) ppb by weight
Gallium: crystal structure
Gallium: biological data
Human abundance by weight: (no data) ppb by weight
Gallium apparently has no biological role but is said to stimulate the metabolism. Gallium compounds appear not to be particularly toxic.
Gallium: uses
Gallium: reactions
Reactions of gallium as the element with air, water, halogens, acids, and bases where known.
Gallium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of gallium where known.
Gallium: compound properties
Bond strengths; lattice energies of gallium halides, hydrides, oxides (where known); and reduction potentials where known.
Gallium: history
Gallium was discovered by Paul-Emile Lecoq de Boisbaudran in 1875 at France. Origin of name: from the Latin word "Gallia" meaning "France" and perhaps also from the Latin word "gallus", (the cock, a translation of Lecoq, the discoveror of gallium).Gallium: isotopes
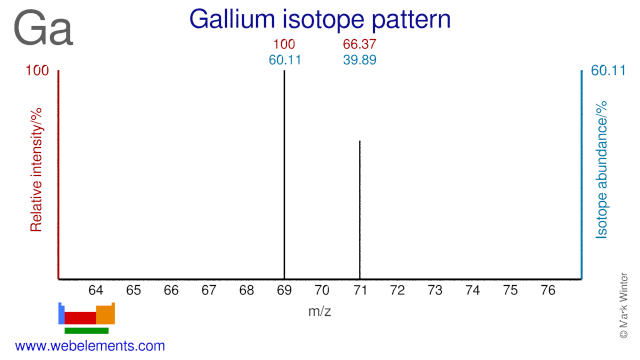
Gallium has two stable isotopes and both are used in nuclear medicine and physics. Ga-69 is used for production of the radioisotope Ge-68. This isotope is used for so-called Ge-68/Ga-68 generators. The Ga-68 that is created from the decay of Ge-68 is used as a PET isotope. Ga-71 has been used to study the behavior of solar neutrinos and it is also used in NMR studies.
Gallium: isolation
Isolation: gallium is normally a byproduct of the manufacture of aluminium. The purification of bauxite by the Bayer process results in concentration of gallium in the alkaline solutions from an aluminium:gallum ratio from 5000 to 300. Electrolysis using a mercury electrode gives a further concentration and further electrolysis using a stainless steel cathode of the resulting sodium gallate affords liquid gallium metal.
Very pure gallium requires a number of further processes ending with zone refining to make very pure gallium metal.