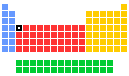
Scandium - 21Sc: the essentials
- Name: scandium
- Symbol: Sc
- Atomic number: 21
- Relative atomic mass (Ar): 44.955907 (4)
- Standard state: solid at 298 K
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table: 3
- Group name: (none)
- Period in periodic table: 4
- Block in periodic table: d
- Shell structure: 2.8.9.2
- CAS Registry: 7440-20-2
Scandium atoms have 21 electrons and the shell structure is 2.8.9.2. The ground state electronic configuration of neutral scandium is [Ar].3d1.4s2 and the term symbol of scandium is 2D3/2.
Scandium: description
Scandium is a silvery-white metal which develops a slightly yellowish or pinkish cast upon exposure to air. It is relatively soft, and resembles yttrium and the rare-earth metals more than it resembles aluminium or titanium. Scandium reacts rapidly with many acids.
Scandium is apparently a much more abundant element in the sun and certain stars than on earth.

Image adapted with permission from Prof James Marshall Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Scandium: physical properties
Density of solid: 2985 kg m-3
Molar volume: 15.00 cm3
Thermal conductivity: 16 W m‑1 K‑1
Scandium: heat properties
Melting point: 1814 [1541 °C (2806 °F)] K
Boiling point: 3103 [2830 °C (5126 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Scandium: atom sizes
Atomic radius (empirical): 160 pm
Molecular single bond covalent radius: 148 (coordination number 3) ppm
van der Waals radius: 258 ppm
Scandium: electronegativities
Pauling electronegativity: 1.36 (Pauling units)
Allred Rochow electronegativity: 1.20 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Scandium: orbital properties
First ionisation energy: 633.09 kJ mol‑1
Second ionisation energy: 1234.99 kJ mol‑1
Third ionisation energy: 2388.67 kJ mol‑1
Scandium: abundances
Universe: 30 ppb by weight
Crustal rocks: 26000 ppb by weight
Human: (no data) ppb by weight
Scandium: crystal structure
Scandium: biological data
Human abundance by weight: (no data) ppb by weight
Scandium has no biological role.
Scandium: uses
Scandium: reactions
Reactions of scandium as the element with air, water, halogens, acids, and bases where known.
Scandium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of scandium where known.
Scandium: compound properties
Bond strengths; lattice energies of scandium halides, hydrides, oxides (where known); and reduction potentials where known.
Scandium: history
Scandium was discovered by Lars Fredrik Nilson in 1879 at Sweden. Origin of name: from the Latin word "Scandia" meaning "Scandinavia".Scandium: isotopes
Scandium: isolation
Isolation: preparation of metallic samples of scandium is not normally necessary given that it is commercially avaialable. In practice littel scandium is produced. The mineral thortveitite contains 35-40% Sc2O3 is used to produce scandium metal but another important source is as a byproduct from uranium ore processing, even though these only contain 0.02% Sc2O3.