Thallium - 81Tl: the essentials
- Name: thallium
- Symbol: Tl
- Atomic number: 81
- Relative atomic mass (Ar): 204.38 ±0.1. Range: [204.382, 204.385]
- Standard state: solid at 298 K
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table: 13
- Group name: (none)
- Period in periodic table: 6
- Block in periodic table: p
- Shell structure: 2.8.18.32.18.3
- CAS Registry: 7440-28-0
Thallium atoms have 81 electrons and the shell structure is 2.8.18.32.18.3. The ground state electronic configuration of neutral thallium is [Xe].4f14.5d10.6s2.6p1 and the term symbol of thallium is 2P1/2.
Thallium: description
When freshly exposed to air, thallium exhibits a metallic lustre, but soon develops a bluish-grey tinge, resembling lead in appearance. A heavy oxide builds up on thallium if left in air, and in the presence of water the hydroxide is formed. The metal is very soft and malleable. It can be cut with a knife.
The element and its compounds are toxic and should be handled carefully. Thallium may cause cancer.

Cartoon by Nick D Kim ([Science and Ink], used by permission).
Thallium: physical properties
Density of solid: 11850 kg m-3
Molar volume: 17.22 cm3
Thermal conductivity: 46 W m‑1 K‑1
Thallium: heat properties
Melting point: 577 [304 °C (579 °F)] K
Boiling point: 1746 [1473 °C (2683 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Thallium: atom sizes
Atomic radius (empirical): 190 pm
Molecular single bond covalent radius: 144 (coordination number 3) ppm
van der Waals radius: 260 ppm
Thallium: electronegativities
Pauling electronegativity: 1.62 (Pauling units)
Allred Rochow electronegativity: 1.44 (Pauling units)
Mulliken-Jaffe electronegativity: 1.96 (sp2 orbital)
Thallium: orbital properties
First ionisation energy: 589.36 kJ mol‑1
Second ionisation energy: 1971.03 kJ mol‑1
Third ionisation energy: 2880.28 kJ mol‑1
Thallium: abundances
Universe: 0.5 ppb by weight
Crustal rocks: 530 ppb by weight
Human: (no data) ppb by weight
Thallium: crystal structure
Thallium: biological data
Human abundance by weight: (no data) ppb by weight
Thallium has no biological role. Thallium compounds are extremely toxic. Their effects are cumulative and they can be absorbed though the skin. Thallium poisoning takes several days to act and it affects the nervous system.
Thallium: uses
Thallium: reactions
Reactions of thallium as the element with air, water, halogens, acids, and bases where known.
Thallium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of thallium where known.
Thallium: compound properties
Bond strengths; lattice energies of thallium halides, hydrides, oxides (where known); and reduction potentials where known.
Thallium: history
Thallium was discovered by Sir William Crookes in 1861 at England. Origin of name: from the Greek word "thallos" meaning "green twig" or green shoot.Thallium: isotopes
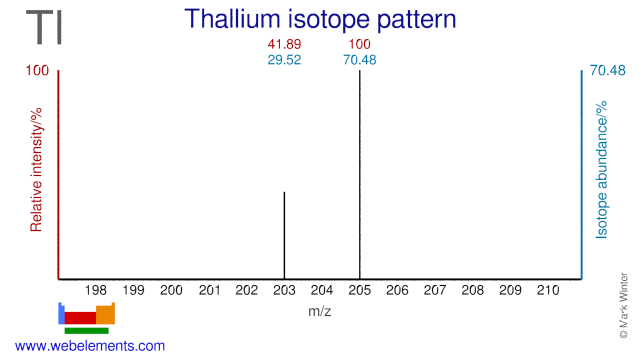
Thallium has two stable isotopes and one of these, Tl-203, is used to produce one of the (workhorses( of nuclear medicine: Tl-201. Tl-201 is used extensively for imaging and in particular for perfusion tests of the myocardium. These tests are done to determine the damage to the heart from a heart attack or from heart diseases. Tl205 has been proposed as an alternative target for the production of Tl-201. Tl-205 is also used in nuclear magnetic resonance research.
Thallium: isolation
Isolation: thallium metal would not normally be made in the laboratory as it is available commercially. Crude thallium is present as a component in flue dust along with arsenic, cadmium, indium, germanium, lead, nickel, selenium, tellurium, and zinc. This is done by dissolving in dilute acid, precipitating out lead sulphate, and then adding HCl to precipitate thallium chloride, TlCl. Further purification can be achieve by electrolysis of soluble thallium salts.