Chlorine - 17Cl: the essentials
- Name: chlorine
- Symbol: Cl
- Atomic number: 17
- Relative atomic mass (Ar): 35.45 ±0.01. Range: [35.446, 35.457] m [see notes g m]
- Standard state: gas at 298 K
- Appearance: yellowish green
- Classification: Non-metallic
- Group in periodic table: 17
- Group name: Halogen
- Period in periodic table: 3
- Block in periodic table: p
- Shell structure: 2.8.7
- CAS Registry: 7782-50-5
Chlorine atoms have 17 electrons and the shell structure is 2.8.7. The ground state electronic configuration of neutral chlorine is [Ne].3s2.3p5 and the term symbol of chlorine is 2P3/2.
Chlorine: description
Chlorine is a greenish yellow gas which combines directly with nearly all elements. Chlorine is a respiratory irritant. The gas irritates the mucous membranes and the liquid burns the skin. As little as 3.5 ppm can be detected as an odour, and 1000 ppm is likely to be fatal after a few deep breaths. It was used as a war gas in 1915. It is not found in a free state in nature, but is found commonly as NaCl (solid or seawater).
Chlorine: physical properties
Density of solid: 2030 kg m-3
Molar volume: 17.39 cm3
Thermal conductivity: 0.0089 W m‑1 K‑1
Chlorine: heat properties
Melting point: 171.6 [‑101.5 °C (‑150.7 °F)] K
Boiling point: 239.11 [‑34.04 °C (‑29.27 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Chlorine: atom sizes
Atomic radius (empirical): 100 pm
Molecular single bond covalent radius: 99 (coordination number 1) ppm
van der Waals radius: 182 ppm
Chlorine: electronegativities
Pauling electronegativity: 3.16 (Pauling units)
Allred Rochow electronegativity: 2.83 (Pauling units)
Mulliken-Jaffe electronegativity: 3.10 (14.3% s orbital)
Chlorine: orbital properties
First ionisation energy: 1251.19 kJ mol‑1
Second ionisation energy: 2297.67 kJ mol‑1
Third ionisation energy: 3840 kJ mol‑1
Chlorine: abundances
Universe: 1000 ppb by weight
Crustal rocks: 170000 ppb by weight
Human: 1200000 ppb by weight
Chlorine: crystal structure
Chlorine: biological data
Human abundance by weight: 1200000 ppb by weight
Chlorine as chloride (Cl-) is essential for mammals and plants. Digestive juices in the stomache contain hydrochloric acid.
Chlorine: uses
Chlorine: reactions
Reactions of chlorine as the element with air, water, halogens, acids, and bases where known.
Chlorine: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of chlorine where known.
Chlorine: compound properties
Bond strengths; lattice energies of chlorine halides, hydrides, oxides (where known); and reduction potentials where known.
Chlorine: history
Chlorine was discovered by Carl William Scheele in 1774 at Sweden. Origin of name: from the Greek word "chloros" meaning "pale green".Chlorine: isotopes
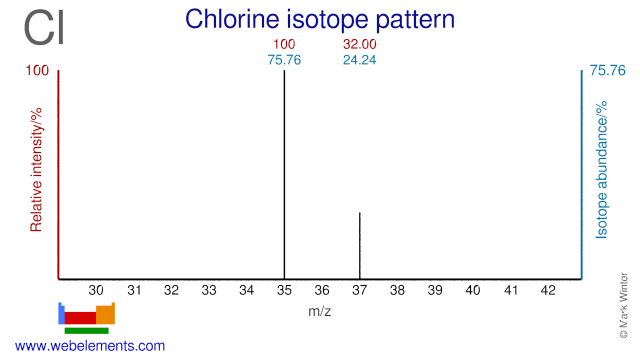
Both Chlorine isotopes, Cl-35 and Cl-37, are used to study the toxicity of environmental pollutant and are usually supplied in the form of NaCl.
Chlorine: isolation
Isolation: it is rarely necessary to make chlorine in the laboratory as it is readily available commercially in cylindes. Chlorine is found largely in seawater where it exists as sodium chloride. It is recovered as a reactive, corrosive, pale green chlorine gas from brine (a solution of sodium chloride in water) by electrolyis. Electrolysis of molten salt, NaCl, also succeeds, in which case the other product is sodium metal rather than sodium hydroxide.
Na+ + Cl- + H2O → Na+ + 1/2Cl2 + 1/2H2 + OH-
In the laboratory under carefully controlled conditions, chlorine can be made by the action of an oxidizing agent such as manganese dioxide, MnO2, upon concentrated hydrochloric acid - the same reaction used by Scheele in 1774 when discovering chlorine.
MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O