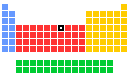
Cobalt - 27Co: the essentials
- Name: cobalt
- Symbol: Co
- Atomic number: 27
- Relative atomic mass (Ar): 58.933194 (3)
- Standard state: solid at 298 K
- Appearance: lustrous, metallic, greyish tinge
- Classification: Metallic
- Group in periodic table: 9
- Group name: (none)
- Period in periodic table: 4
- Block in periodic table: d
- Shell structure: 2.8.15.2
- CAS Registry: 7440-48-4
Cobalt atoms have 27 electrons and the shell structure is 2.8.15.2. The ground state electronic configuration of neutral cobalt is [Ar].3d7.4s2 and the term symbol of cobalt is 4F9/2.
Cobalt: description
Cobalt is a brittle, hard, silver-grey transition metal with magnetic properties similar to those of iron (ferromagnetic). Cobalt is present in meteorites. Ore deposits are found in Zaire, Morocco and Canada. The isotope cobalt-60 (60Co) is an artificially produced isotope used as a source of γ rays (its high energy radiation is useful for sterilisation in medicine and of foods). Cobalt salts colour glass a beautiful deep blue colour. Cobalt compounds are important catalysts in a number of industrial processes. Cobalt is required in small amounts for life and is the only metal found in vitamins (cobalt is the critical component of vitamin B12.
Marmite, which we eat here in England and love it or hate it is a source of vitamin B12, actually a cobalt complex. The equivalent, but blander, in Australia is Vegemite. Marmite is available in the USA. Try mixing it with peanut butter.

Cobalt: physical properties
Density of solid: 8900 kg m-3
Molar volume: 6.67 cm3
Thermal conductivity: 100 W m‑1 K‑1
Cobalt: heat properties
Melting point: 1768 [1495 °C (2723 °F)] K
Boiling point: 3200 [2927 °C (5301 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Cobalt: atom sizes
Atomic radius (empirical): 135 pm
Molecular single bond covalent radius: 111 (coordination number 4) ppm
van der Waals radius: 240 ppm
Cobalt: electronegativities
Pauling electronegativity: 1.88 (Pauling units)
Allred Rochow electronegativity: 1.70 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Cobalt: orbital properties
First ionisation energy: 760.40 kJ mol‑1
Second ionisation energy: 1648.39 kJ mol‑1
Third ionisation energy: 3232.3 kJ mol‑1
Cobalt: abundances
Universe: 3000 ppb by weight
Crustal rocks: 30000 ppb by weight
Human: 20 ppb by weight
Cobalt: crystal structure
Cobalt: biological data
Human abundance by weight: 20 ppb by weight
Cobalt salts in small amounts are essential to many life forms, including humans. It is at the core of a vitamin called vitamin-B12. Grazing animals do not to do well in areas where there is little cobalt in the soil.
Cobalt: uses
Cobalt: reactions
Reactions of cobalt as the element with air, water, halogens, acids, and bases where known.
Cobalt: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of cobalt where known.
Cobalt: compound properties
Bond strengths; lattice energies of cobalt halides, hydrides, oxides (where known); and reduction potentials where known.
Cobalt: history
Cobalt was discovered by Georg Brandt in 1735 at Sweden. Origin of name: from the German word "kobald" meaning "goblin" or evil spirit.Cobalt: isotopes
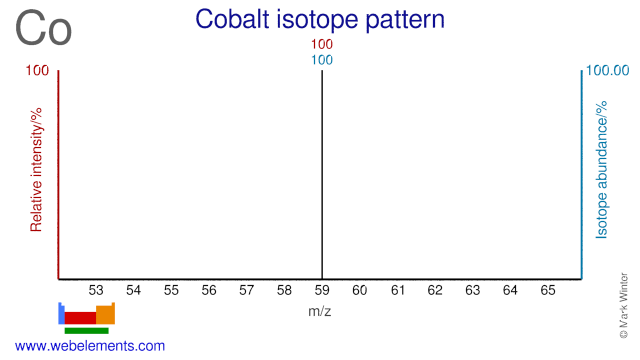
Naturally occurring cobalt consists of a single stable isotope: 59Co. Cobalt-59 has spin 7/2. The usual NMR reference is K3[Co(CN)6] in D2O. Cobalt-60 is an artifical isotope, and is an important γ-ray source.
Cobalt: isolation
Isolation: it is not normally necessary to make cobalt in the laboratory as it is available readily commercially. Many ores contain cobalt but not many are of economic importance. These include the sulphides and arsenides linnaeite, Co3S4, cobaltite, CoAsS, and smaltite, CoAs2. Industrially, however, it is normally produced as a byproduct from the produstion of copper, nickel, and lead.
Normally the ore is "roasted" to form a mixture of metals and metal oxides. Treatment with sulphuric acid leaves metallic copper as a residue and disolves out iron, cobalt, and nickel as the sulphates. Iron is obtained by precipitation with lime (CaO) while cobalt is produced as the hydroxide by precipitation with sodium hypochlorite (NaOCl)
2Co2+(aq) + NaOCl(aq) + 4OH-(aq) + H2O → 2Co(OH)3(s) + NaCl(aq)
The trihydroxide Co(OH)3 is heated to form the oxide and then reduced with carbon (as charcoal) to form cobalt metal.
2Co(OH)3 (heat) → Co2O3 + 3H2O
2Co2O3 + 3C → Co + 3CO2