Silicon - 14Si: the essentials
- Name: silicon
- Symbol: Si
- Atomic number: 14
- Relative atomic mass (Ar): 28.085 ±0.01. Range: [28.084, 28.086] [see note r]
- Standard state: solid at 298 K
- Appearance: dark grey with a bluish tinge
- Classification: Semi-metallic
- Group in periodic table: 14
- Group name: (none)
- Period in periodic table: 3
- Block in periodic table: p
- Shell structure: 2.8.4
- CAS Registry: 7440-21-3
Silicon atoms have 14 electrons and the shell structure is 2.8.4. The ground state electronic configuration of neutral silicon is [Ne].3s2.3p2 and the term symbol of silicon is 3P0.
Silicon: description
Silicon is present in the sun and stars and is a principal component of a class of meteorites known as aerolites. Silicon makes up 25.7% of the earth's crust by weight, and is the second most abundant element, exceeded only by oxygen. It is found largely as silicon oxides such as sand (silica), quartz, rock crystal, amethyst, agate, flint, jasper and opal. Silicon is found also in minerals such as asbestos, feldspar, clay and mica.
Silicon is important in plant and animal life. Diatoms in both fresh and salt water extract silica from the water to use as a component of their cell walls. Silicon is an important ingredient in steel. Silicon carbide is one of the most important abrasives. Workers in environments where silicaceous dust is breathed may develop a serious lung disease known as silicosis.
Hydrolysis and condensation of substituted chlorosilanes can be used to produce a very great number of polymeric products, or silicones. These range from liquids to hard, glasslike solids with many useful properties.
Elemental silicon transmits more than 95% of all wavelengths of infrared and and has been used in lasers to produce coherent light at 456 nm.
Silicon: physical properties
Density of solid: 2330 kg m-3
Molar volume: 12.06 cm3
Thermal conductivity: 150 W m‑1 K‑1
Silicon: heat properties
Melting point: 1687 [1414 °C (2577 °F)] K
Boiling point: 3173 [2900 °C (5252 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Silicon: atom sizes
Atomic radius (empirical): 110 pm
Molecular single bond covalent radius: 116 (coordination number 4) ppm
van der Waals radius: 219 ppm
Silicon: electronegativities
Pauling electronegativity: 1.90 (Pauling units)
Allred Rochow electronegativity: 1.74 (Pauling units)
Mulliken-Jaffe electronegativity: 2.28 (sp3 orbital)
Silicon: orbital properties
First ionisation energy: 786.52 kJ mol‑1
Second ionisation energy: 1577.13 kJ mol‑1
Third ionisation energy: 3231.58 kJ mol‑1
Silicon: abundances
Universe: 700000 ppb by weight
Crustal rocks: 270000000 ppb by weight
Human: 260000 ppb by weight
Silicon: crystal structure
Silicon: biological data
Human abundance by weight: 260000 ppb by weight
Silicon is probably essential in higher plants and perhaps to mammals. Diatoms, some protozoa, some sponges, and some plants use silicon dioxide (SiO2) as a structural material. Silicon is known to be required by chicks and rats for growth and skeletal development. Silicon is not particularly toxic but finely divided silicates or silica cause major damage to lungs.
Silicon: uses
Silicon: reactions
Reactions of silicon as the element with air, water, halogens, acids, and bases where known.
Silicon: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of silicon where known.
Silicon: compound properties
Bond strengths; lattice energies of silicon halides, hydrides, oxides (where known); and reduction potentials where known.
Silicon: history
Silicon was discovered by Jöns Jacob Berzelius in 1824 at Sweden. Origin of name: from the Latin word "silicis" meaning "flint".Silicon: isotopes
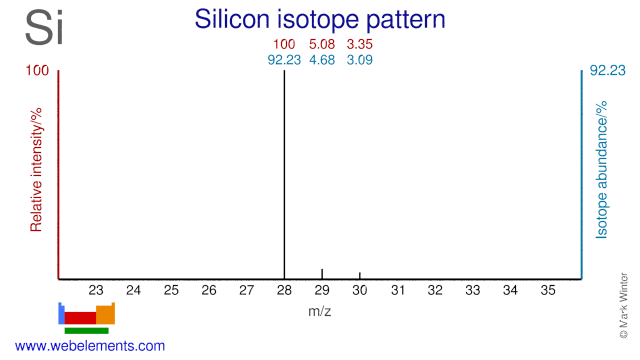
Silicon isotopes are used in a variety of applications. Si-28 has been suggested to improve the thermal conductivity of semiconductors. Si-29 is used extensively in NMR spectroscopy. Si-30 has been used to produce the radioisotope Si-31. Si-30 has also been used to study the self-diffusivity of Silicon and it has been used to study the isotope effect on superconductivity.
Silicon: isolation
Isolation: there is normally no need to make silicon in the laboratory as it is readily available commercially. Silicon is readily available through the treatment of silica, SiO2, with pure graphite (as coke) in an electric furnace.
SiO2 + 2C → Si + 2CO
Under these conditions, silicon carbide, SiC, can form. However, provided the amount of SiO2 is kept high, silicon carbide may be eliminated.
2SiC + SiO2 → 3Si + 2CO
Very pure silicon can be made by the reaction of SiCl4 with hydrogen, followed by zone refining of the resultant silicon.
SiCl4 + 2H2 → Si + 4HCl