Cobalt - 27Co: physical properties
Cobalt is a brittle, hard, silver-grey transition metal with magnetic properties similar to those of iron (it is ferromagnetic). It has a high melting point and is hard-wearing even at high temperatures. Its alloys also possess useful properties and so it finds use in high speed steels and cutting tools for instance. The physical properties of cobalt resulted in its use in a cobalt-chromium-molybdenum alloy (Vitallium, 1937) that is strong, has a good corrosion resistance and is tolerated by the body. These days cobalt alloys are used less as they are heavy.
Density properties
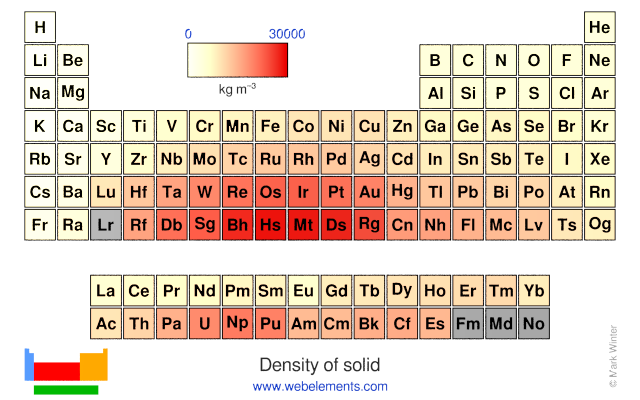
 Density of solid: 8900 kg m‑3
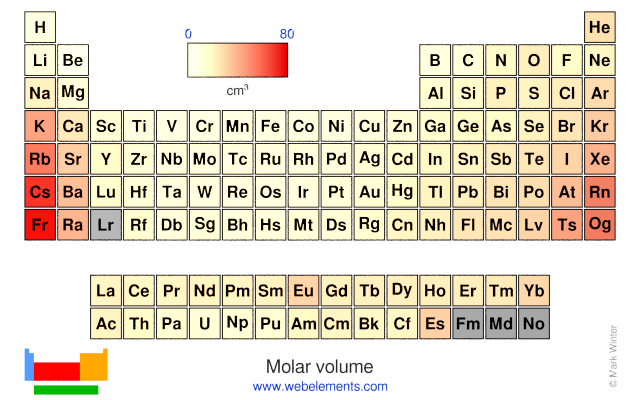
Density of solid: 8900 kg m‑3 Molar volume: 6.67 cm3
Molar volume: 6.67 cm3
Elastic properties
 Young's modulus: 209 GPa
Young's modulus: 209 GPa Rigidity modulus: 75 GPa
Rigidity modulus: 75 GPa Bulk modulus: 180 GPa
Bulk modulus: 180 GPa Poisson's ratio: 0.31 (no units)
Poisson's ratio: 0.31 (no units)
Hardnesses
 Mineral hardness: 5.0 (no units)
Mineral hardness: 5.0 (no units) Brinell hardness: 700 MN m-2
Brinell hardness: 700 MN m-2 Vickers hardness: 1043 MN m-2
Vickers hardness: 1043 MN m-2
Electrical properties
 Electrical resistivity: 6 × 10‑8 Ω m; or mΩ cm
Electrical resistivity: 6 × 10‑8 Ω m; or mΩ cm
Heat and conduction
 Thermal conductivity: 100 W m‑1 K‑1
Thermal conductivity: 100 W m‑1 K‑1 Coefficient of linear thermal expansion: 13.0 × 10‑6 K‑1
Coefficient of linear thermal expansion: 13.0 × 10‑6 K‑1
Optical properties

The image above is a virtual representation of cobalt metal calculated by Patrick Callet using the complex diectric function of the element only.
 Reflectivity: 67 %
Reflectivity: 67 % Refractive index: (no data) (no units)
Refractive index: (no data) (no units)
Acoustic properties
 Velocity of sound: 4720 m s‑1
Velocity of sound: 4720 m s‑1