Krypton - 36Kr: the essentials
- Name: krypton
- Symbol: Kr
- Atomic number: 36
- Relative atomic mass (Ar): 83.798 (2) g,m [see notes g m]
- Standard state: gas at 298 K
- Appearance: colourless
- Classification: Non-metallic
- Group in periodic table: 18
- Group name: Noble gas
- Period in periodic table: 4
- Block in periodic table: p
- Shell structure: 2.8.18.8
- CAS Registry: 7439-90-9
Krypton atoms have 36 electrons and the shell structure is 2.8.18.8. The ground state electronic configuration of neutral krypton is [Ar].3d10.4s2.4p6 and the term symbol of krypton is 1S0.
Krypton: description
Krypton is present in the air at about 1 ppm. The atmosphere of Mars contains a little (about 0.3 ppm) of krypton. It is characterised by its brilliant green and orange spectral lines. The spectral lines of krypton are easily produced and some are very sharp. In 1960 it was internationally agreed that the fundamental unit of length, the metre, should be defined as 1 m = 1,650,763.73 wavelengths (in vacuo) of the orange-red line of Kr-33.
Under normal conditions krypton is colourless, odourless, fairly expensive gas. Solid krypton is a white crystalline substance with a face-centered cubic structure which is common to all the "rare gases". Krypton difluoride, KrF2, has been prepared in gram quantities and can be made by several methods.

Image adapted with permission from Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Krypton: physical properties
Density of solid: 2155 kg m-3
Molar volume: 27.99 cm3
Thermal conductivity: 0.00943 W m‑1 K‑1
Krypton: heat properties
Melting point: 115.79 [‑157.36 °C (‑251.25 °F)] K
Boiling point: 119.93 [‑153.22 °C (‑243.8 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Krypton: atom sizes
Atomic radius (empirical): (no data) pm
Molecular single bond covalent radius: 117 (coordination number 1,2) ppm
van der Waals radius: 225 ppm
Krypton: electronegativities
Pauling electronegativity: 3.00 (Pauling units)
Allred Rochow electronegativity: 2.94 (Pauling units)
Mulliken-Jaffe electronegativity: 3.00 (12.5% s orbital)
Krypton: orbital properties
First ionisation energy: 1350.76 kJ mol‑1
Second ionisation energy: 2350.37 kJ mol‑1
Third ionisation energy: 3457.8 kJ mol‑1
Krypton: abundances
Universe: 40 ppb by weight
Crustal rocks: 0.15 ppb by weight
Human: (no data) ppb by weight
Krypton: crystal structure
Krypton: biological data
Human abundance by weight: (no data) ppb by weight
Krypton has no biological role.
Krypton: uses
Krypton: reactions
Reactions of krypton as the element with air, water, halogens, acids, and bases where known.
Krypton: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of krypton where known.
Krypton: compound properties
Bond strengths; lattice energies of krypton halides, hydrides, oxides (where known); and reduction potentials where known.
Krypton: history
Krypton was discovered by Sir William Ramsay, Morris W. Travers in 1898 at Great Britain. Origin of name: from the Greek word "kryptos" meaning "hidden".Krypton: isotopes
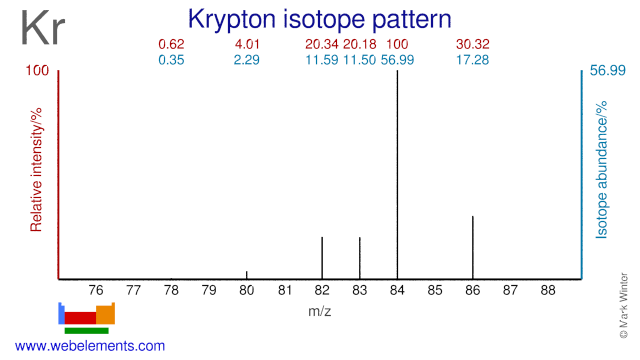
Krypton isotopes are used in various medical and scientific applications. Kr-82 is used for the production of Rb-81/Kr-81m generators. Many of the stable isotopes of Krypton are used in the study of the pulmonary system. Kr-78 can be used for the production of Br-75 although production of Br-75 via Se-76 is more common. Kr-86 has been used to define the standard measure of length: 1 meter is exactly 1,650,763.73 wavelengths of this isotope.
Krypton: isolation
Isolation: krypton is present to a small extent (about 1 ppm by volume) in the atmosphere and is obtained as a byproduct from the liquefaction and separation of air. This would not normally be carried out in the laboratory and krypton is available commercially in cylinders at high pressure.