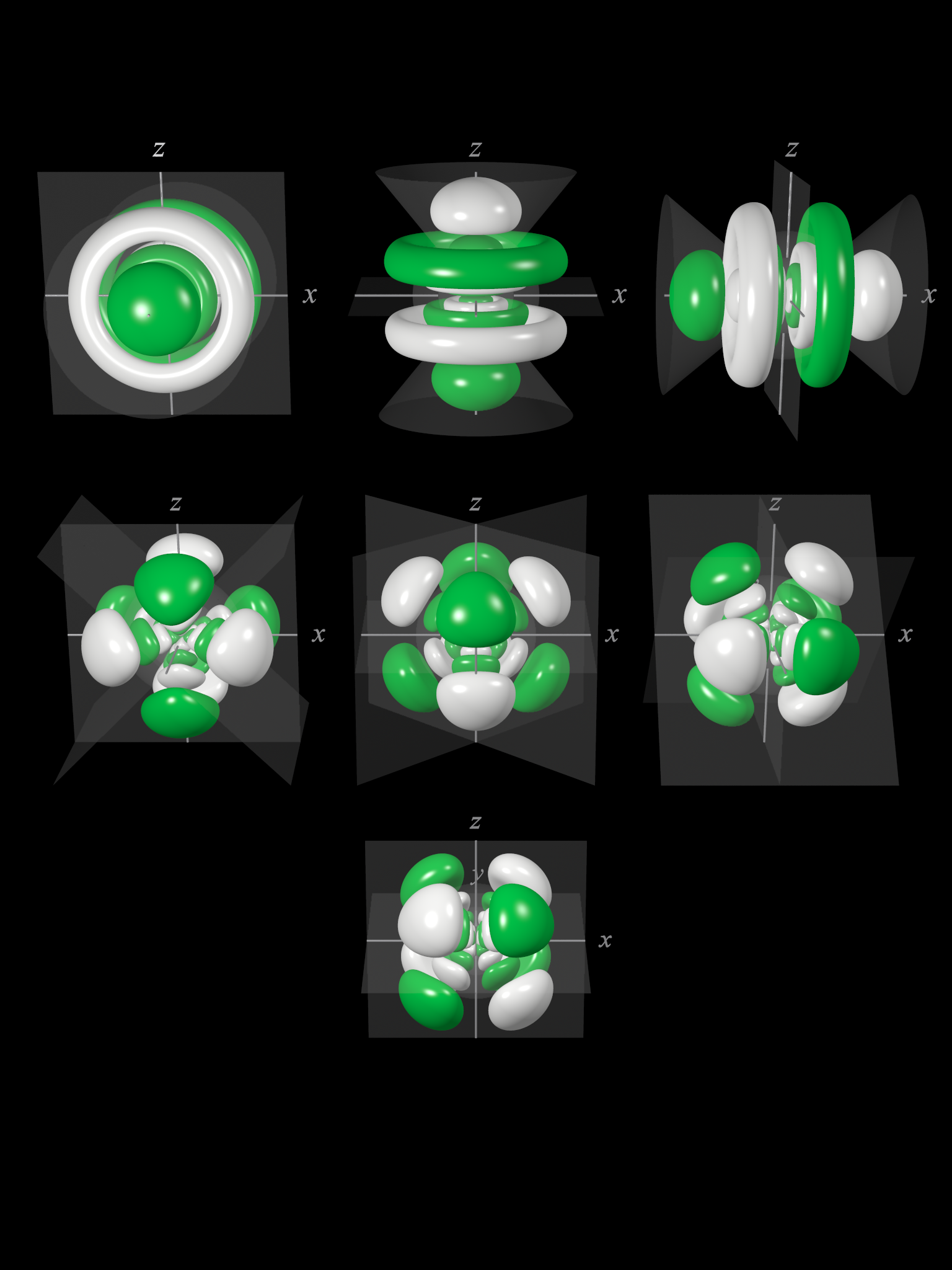
7f atomic orbitals
For any atom, there are seven 7f orbitals. The f-orbitals are unusual in that there are two sets of orbitals in common use. The first set is known as the general set, this page. The second set is the cubic set, this page and these might be appropriate to use if the atom is in a cubic environment, for instance. Three of the orbitals are common to both sets. These are are the 7fxyz, 7fz3, and 7fz(x2-y2) orbitals.
The higher f-orbitals (8f, 9f, ...) are more complex since they have more spherical nodes while the lower orbitals (4f, 5f, and 6f) have fewer.
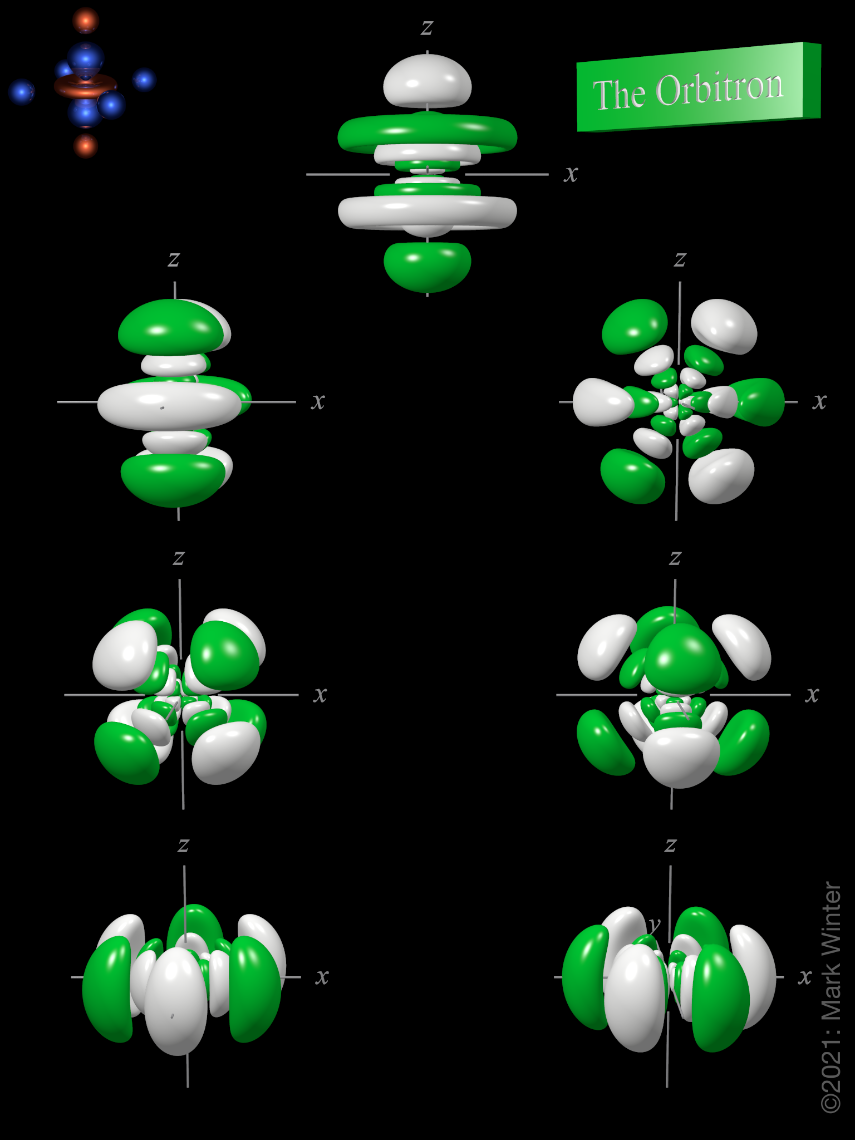
7f atomic orbitals general set
The shape of the seven 7f orbitals (general set). From left to right: (top row) 7fz3, (next to top row) 7fyz2, 7fxz2, (next to bottom row) 7fxyz, and 7fz(x2-y2), (bottom row) 7fy(3x2-y2), 7fx(x2-3y2). For each, the green zones are where the wave functions have positive values and the white zones denote negative values.
In the general set of 7f orbitals, there are four distinct shapes, each of which possess a number of planar and conical nodes. The 7f orbitals possess two radial nodes.
The 7fz3 orbital (top row in the image above) has a planar node in the xy plane and two conical nodes with their exes along the z-axis.
The 7fyz2 and 7fxz2 orbitals (next to top row in the image above) are related to each other by a 90° rotation about the z-axis. At first sight, they are similar in shape to the 7fy(3x2-y2) and 7fx(x2-3y2) orbitals but they are not. While these orbitals contain six lobes, the nodal planes are not at 60° to each other and two of the six lobes are "bean-shaped".
The 7fxyz and 7fz(x2-y2) (next to bottom row in the image above) each have eight lobes and are related to each other by a 45° rotation about the z-axis. Each orbital has three nodal planes, which for the 7fxyz are the xy, xz, and yz planes.
The 7fy(3x2-y2) and 7fx(x2-3y2) orbitals (bottom row in the image above) are related to each other by a 90° rotation about the z-axis. Each orbital has six lobes separated by three nodal planes lying at 60° to each other.
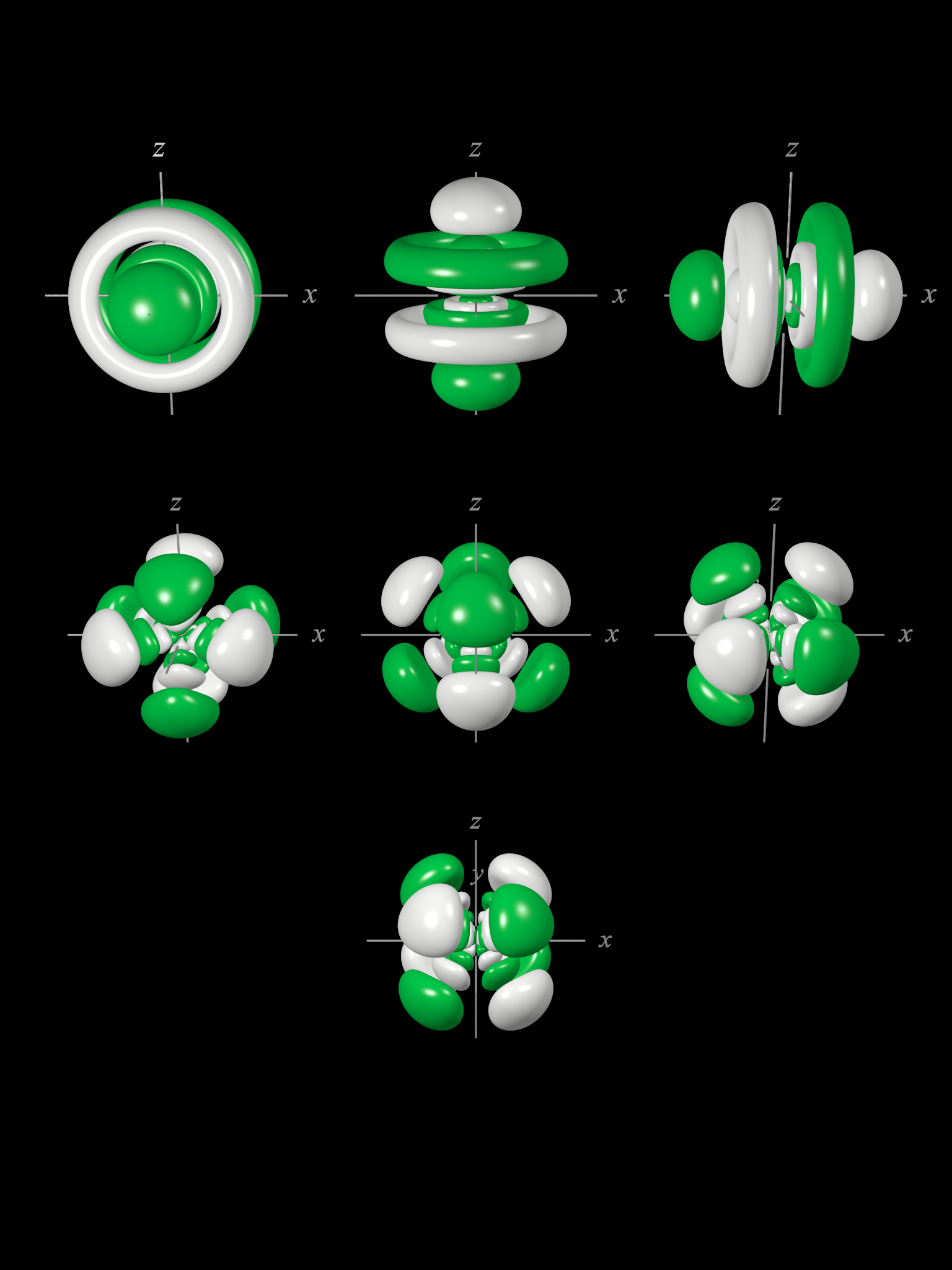
7f atomic orbitals cubic set
The shape of the seven 7f orbitals (cubic set). From left to right: (top row) 7fy3, 7fz3, 7fx3, (middle row) 7fy(z2-x2), 7fz(x2-y2), and 7fx(z2-y2) (bottom row) 7fxyz. For each, the green zones are where the wave functions have positive values and the white zones denote negative values.
In the cubic set of 7f orbitals, there are two distinct shapes, each of which possess a number of planar and conical nodes. None of the 7f orbitals possess radial nodes.
The 7fxyz, 7fx(z2-y2), 7fy(z2-x2), and 7fz(x2-y2) (bottom two rows in the image above) each have eight lobes. The 7fx(z2-y2), 7fy(z2-x2), and 7fz(x2-y2) orbitals are related to each other by 45° rotations about the x, y, and z-axis respectively. Each orbital has three nodal planes, which for the 7fxyz are the xy, xz, and yz planes.
The 7fx3, 7fy3, and 7fz3 orbitals (top row in the image above) has a planar node in the xy plane and two conical nodes orientated along the z-axis. The other two orbitals are related through 90° rotations.
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.