6f atomic orbitals
For any atom, there are seven 6f orbitals. The f-orbitals are unusual in that there are two sets of orbitals in common use. The first set is known as the general set, this page. The second set is the cubic set, this page and these might be appropriate to use if the atom is in a cubic environment, for instance. Three of the orbitals are common to both sets. These are are the 6fxyz, 6fz3, and 6fz(x2-y2) orbitals.
The higher f-orbitals ( 7f) are more complex since they have more spherical nodes while the lower orbitals (5f and 4f) have fewer.
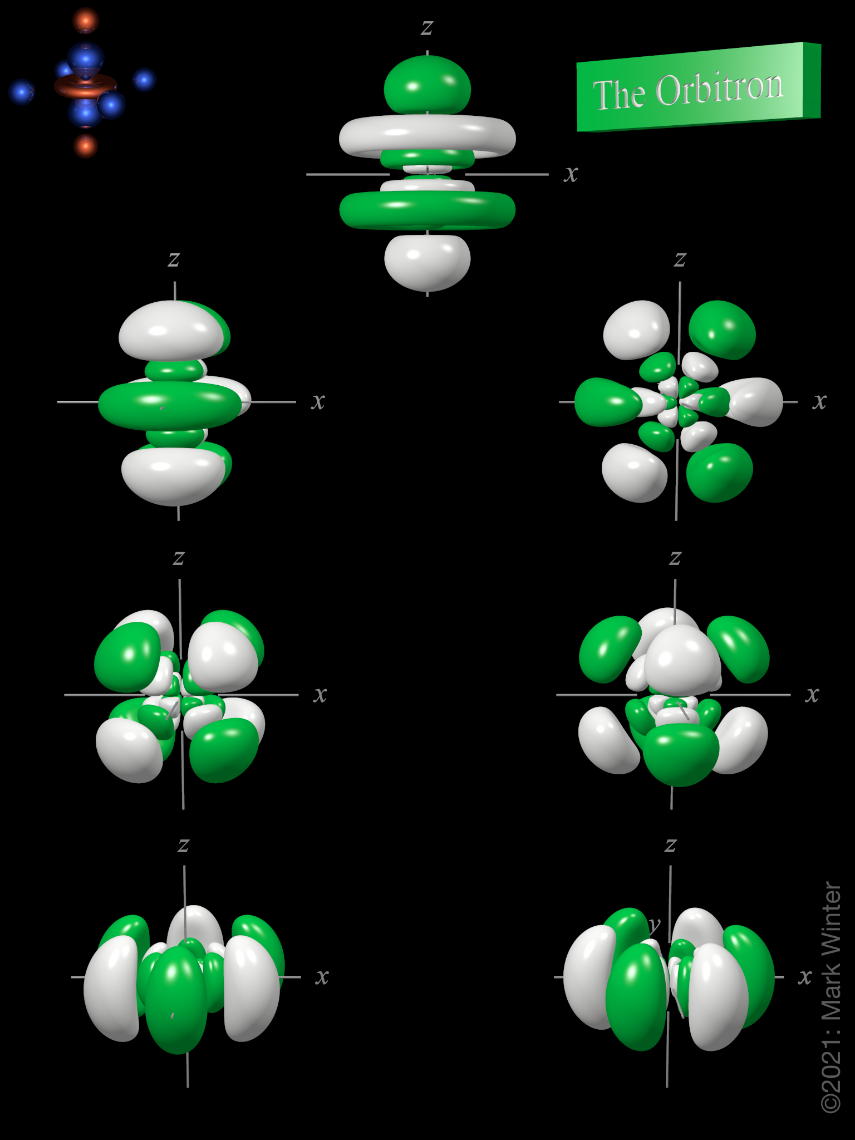
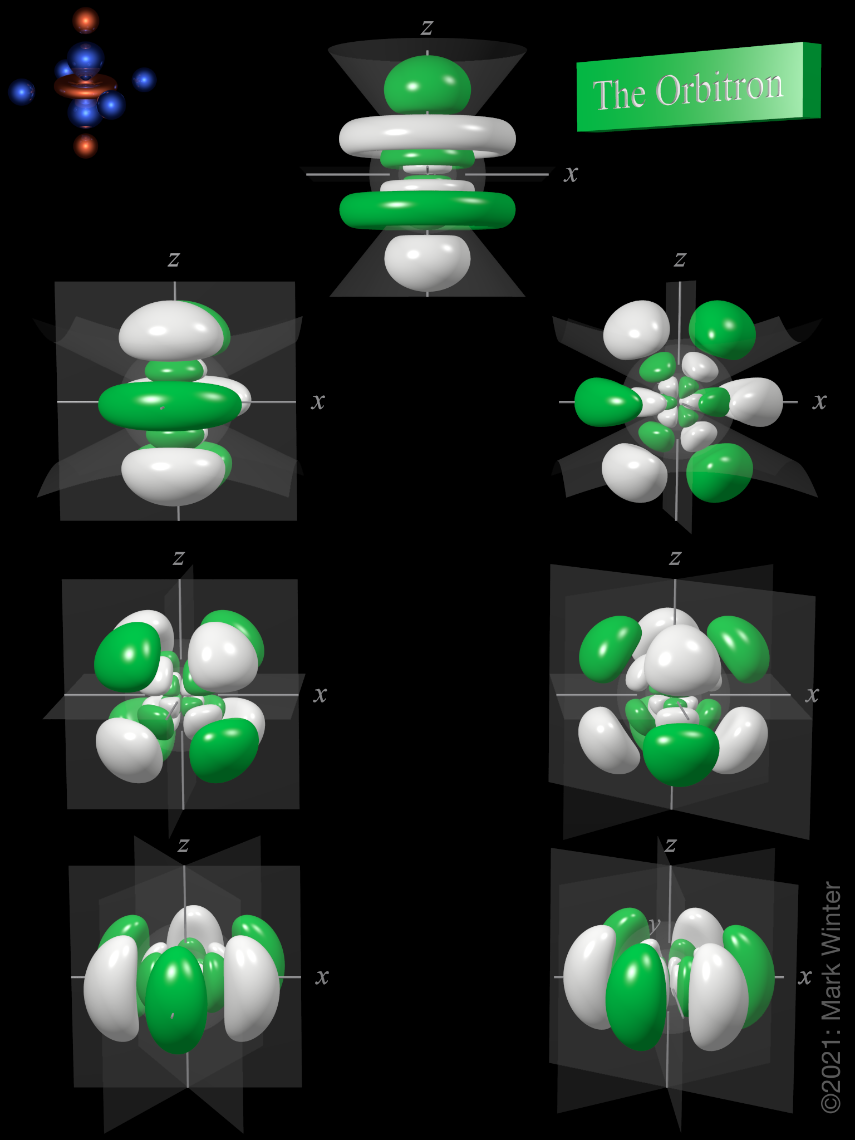
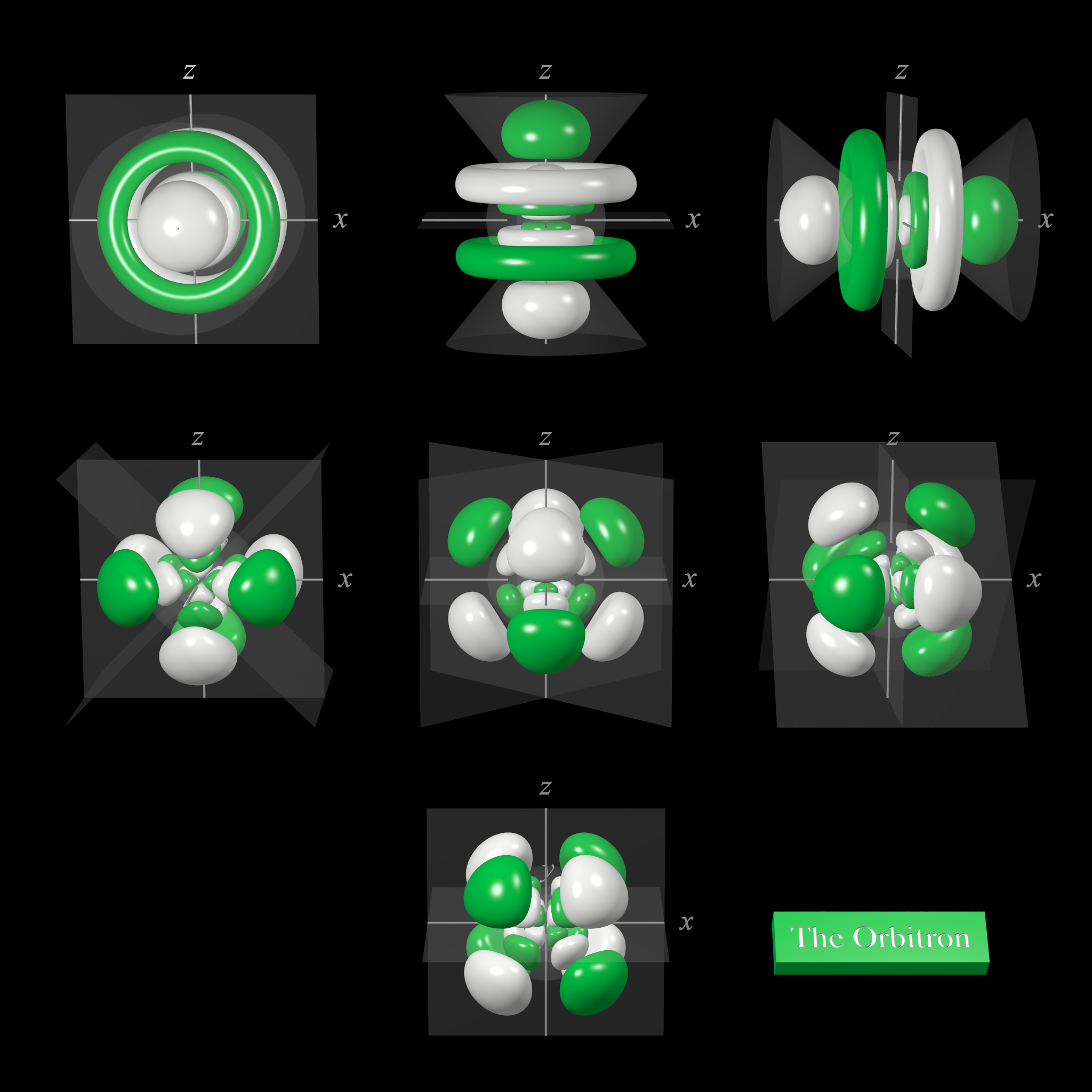
6f atomic orbitals general set
The shape of the seven 6f orbitals (general set). From left to right: (top row) 6fz3, (next to top row) 6fyz2, 6fxz2, (next to bottom row) 6fxyz, and 6fz(x2-y2), (bottom row) 6fy(3x2-y2), 6fx(x2-3y2). For each, the green zones are where the wave functions have positive values and the white zones denote negative values.
In the general set of 6f orbitals, there are four distinct shapes, each of which possess a number of planar and conical nodes. The 6f orbitals possess two radial nodes.
The 6fz3 orbital (top row in the image above) has a planar node in the xy plane and two conical nodes with their exes along the z-axis.
The 6fyz2 and 6fxz2 orbitals (next to top row in the image above) are related to each other by a 90° rotation about the z-axis. At first sight, they are similar in shape to the 6fy(3x2-y2) and 6fx(x2-3y2) orbitals but they are not. While these orbitals contain six lobes, the nodal planes are not at 60° to each other and two of the six lobes are "bean-shaped".
The 6fxyz and 6fz(x2-y2) (next to bottom row in the image above) each have eight lobes and are related to each other by a 45° rotation about the z-axis. Each orbital has three nodal planes, which for the 6fxyz are the xy, xz, and yz planes.
The 6fy(3x2-y2) and 6fx(x2-3y2) orbitals (bottom row in the image above) are related to each other by a 90° rotation about the z-axis. Each orbital has six lobes separated by three nodal planes lying at 60° to each other.
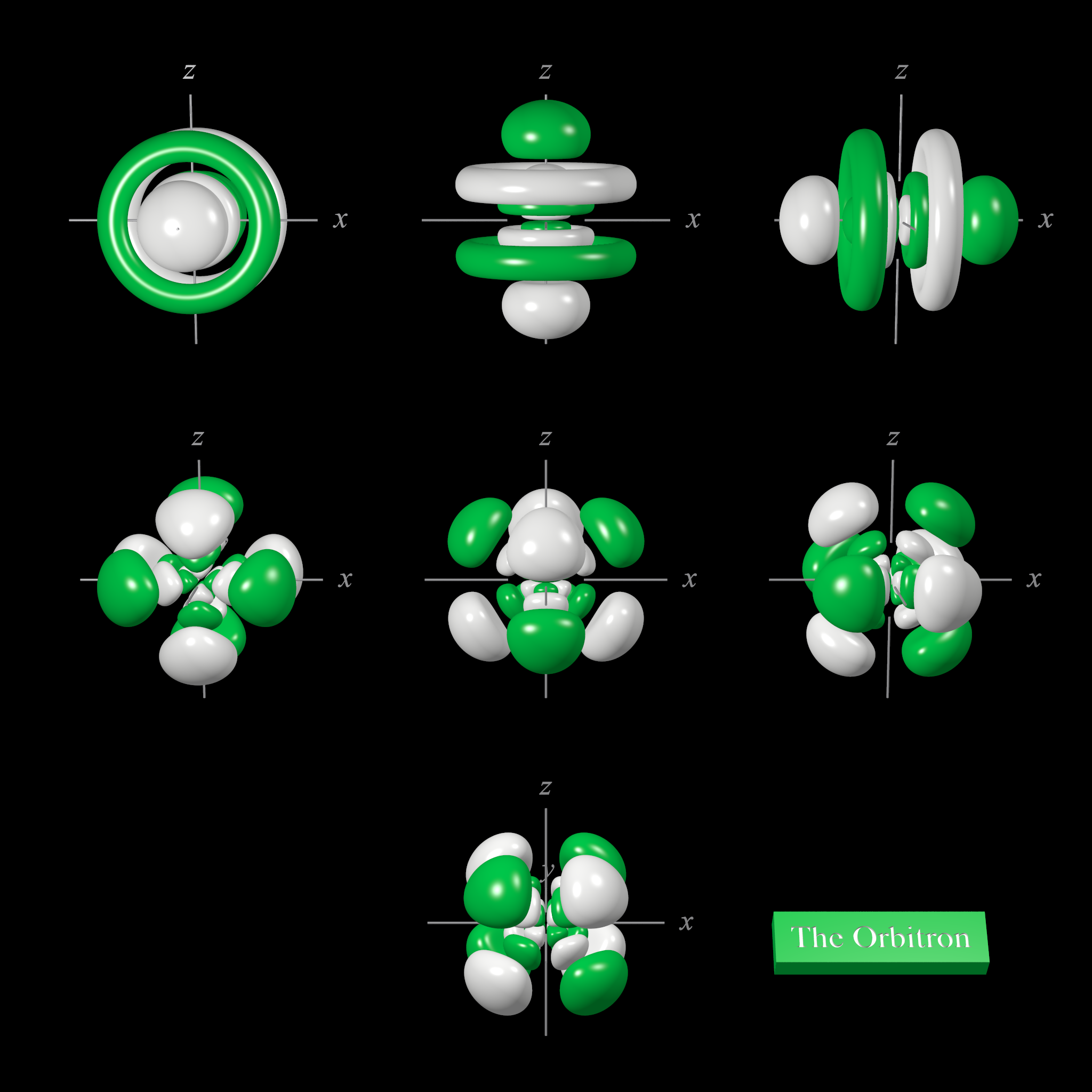
6f atomic orbitals cubic set
The shape of the seven 6f orbitals (cubic set). From left to right: (top row) 6fy3, 6fz3, 6fx3, (middle row) 6fy(z2-x2), 6fz(x2-y2), and 6fx(z2-y2) (bottom row) 6fxyz. For each, the green zones are where the wave functions have positive values and the white zones denote negative values.
In the cubic set of 6f orbitals, there are two distinct shapes, each of which possess a number of planar and conical nodes. None of the 6f orbitals possess radial nodes.
The 6fxyz, 6fx(z2-y2), 6fy(z2-x2), and 6fz(x2-y2) (bottom two rows in the image above) each have eight lobes. The 6fx(z2-y2), 6fy(z2-x2), and 6fz(x2-y2) orbitals are related to each other by 45° rotations about the x, y, and z-axis respectively. Each orbital has three nodal planes, which for the 6fxyz are the xy, xz, and yz planes.
The 6fx3, 6fy3, and 6fz3 orbitals (top row in the image above) has a planar node in the xy plane and two conical nodes orientated along the z-axis. The other two orbitals are related through 90° rotations.
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.