2s atomic orbital
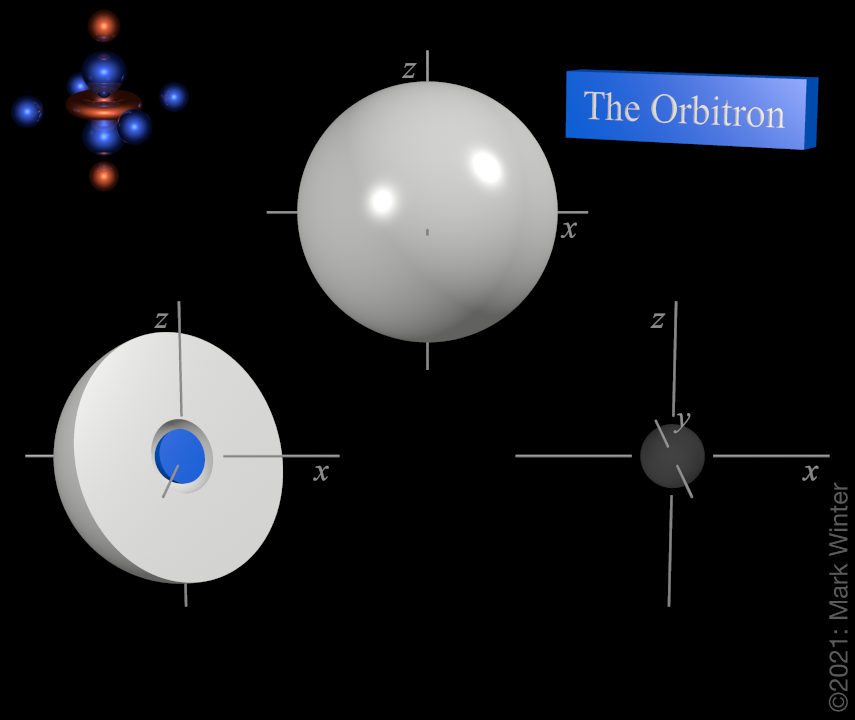
The shape of the 2s orbital. The blue zone is where the wave function has positive values while the white zone is where values are positive.
For any atom there is just one 2s orbital. The image on the top is deceptively simple as the interesting feature is buried within the orbital. That on the left is sliced in half to show that there is a spherical node in the 2s orbital. The shape on the right shows the nodal structure of the 2s-orbital.
The origin of the spherical node becomes clear if we examine the wave equation, which includes a (2 - ρ) term. When (2 - ρ) = 0, then we must have a node. Since for the 2s orbital ρ = 2Zr/2 = Zr (Z = effective nuclear charge, r = radius in atomic units), then the node is at the radius for which (2 - Zr) = 0, that is, r = 2/Z atomic units.
While still spherical, the higher s-orbitals ( 3s, 4s, 5s, 6s, and 7s) are more complex since they have more spherical nodes. While still spherical, the lower s-orbitals ( 1s) is simpler since it has no spherical nodes.
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.