7d atomic orbitals
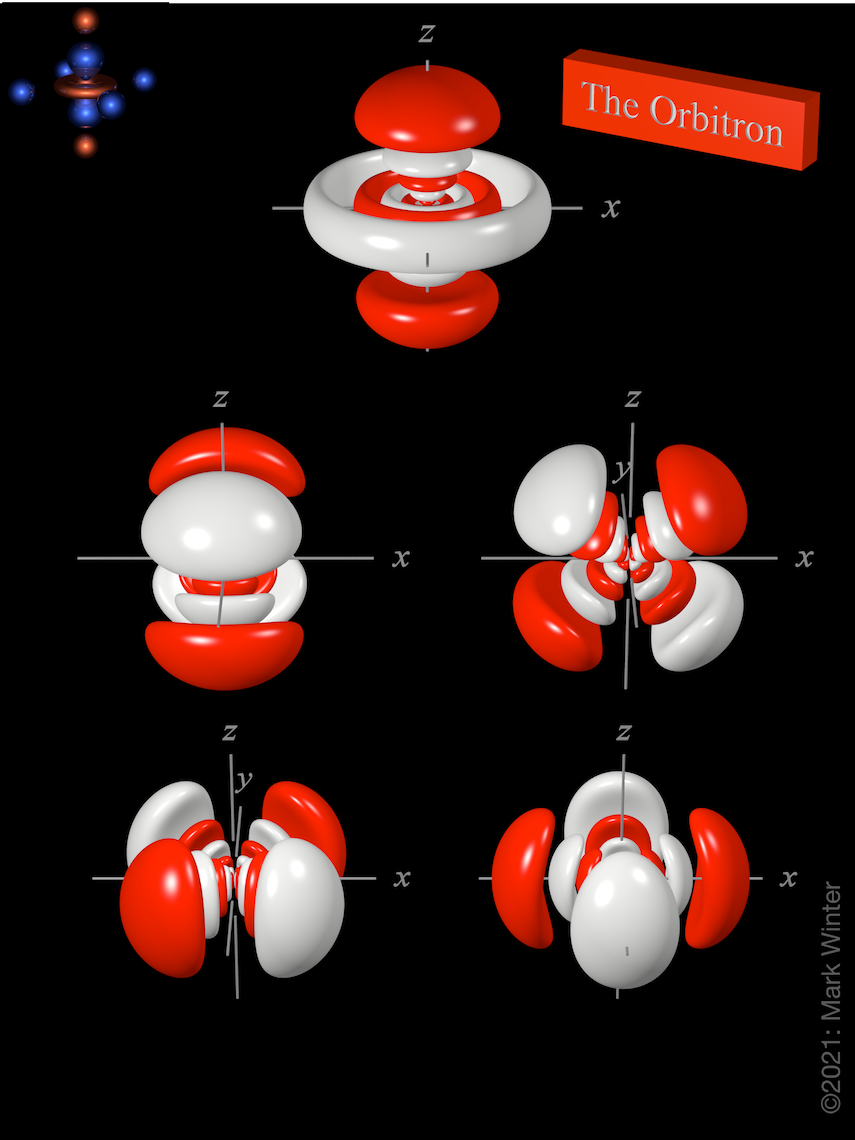
There are five 7d orbitals. These are labelled 7dxy, 7dxz, 7dyz, 7dx2-y2 and 7dz2. The 7dz2 name is an abbreviation for 3d(3z2–r2). Four of these functions have the same shape but are aligned differently in space. The fifth function (7dz2) has a different shape.
The shape of the five 7d orbitals. Top row: 7dz2; centre row from left to right: 7dyz and 7dxz; bottom row: 7dxy and 7dx2-y2. For each, the white zones are where the values of the wave functions are negative while the red zones denote positive values.
There are five 7d orbitals. These are labelled 7dxy, 7dxz, 7dyz, 7dx2-y2 and 7dz2. Four of these functions have the same shape but are aligned differently in space. The fifth function (7dz2) has a different shape.
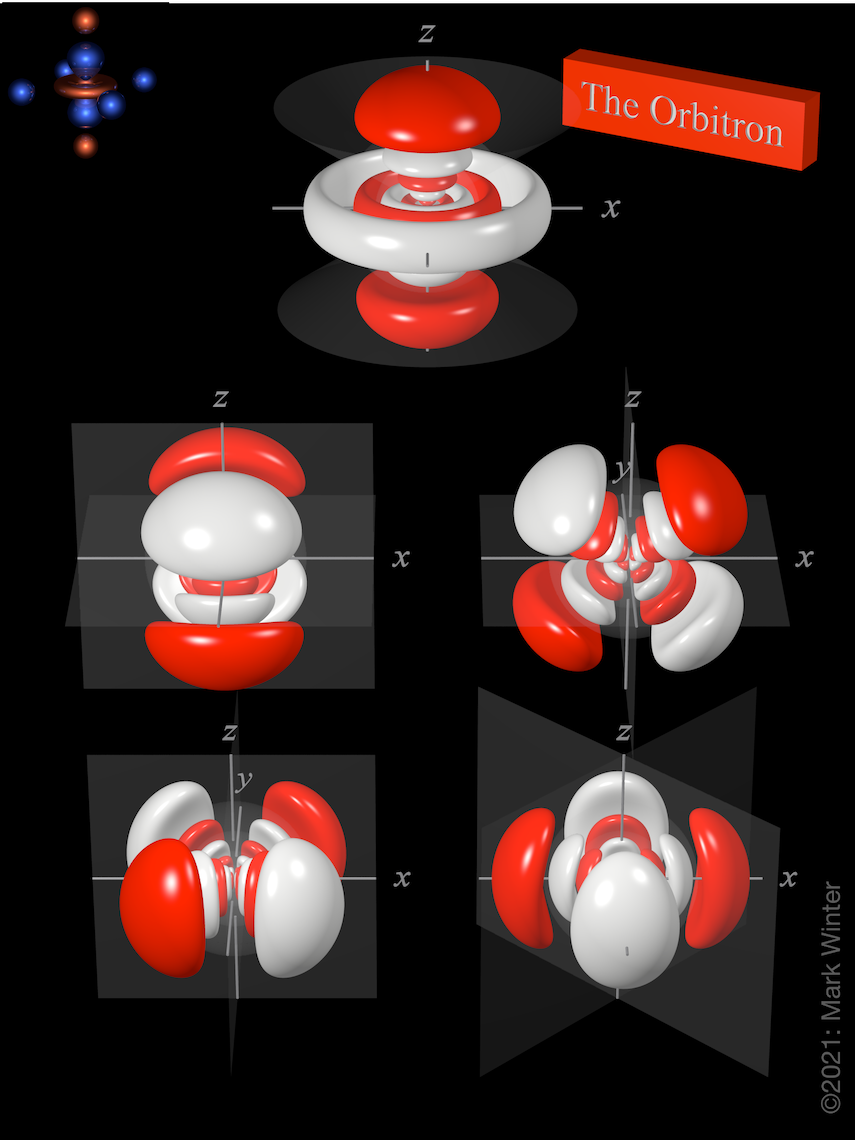
Each 7dxy, 7dxz, 7dyz, and 7dx2-y2 orbital has eight lobes. There are two planar node normal to the axis of the orbital (so the 7dxy orbital has yz and xz nodal planes, for instance). The 7dz2 orbital is a little different and has two conical nodes. In addition, apart from the planar nodes, all five orbitals have three spherical nodes that partition off the small inner lobes. The lower d-orbitals ( 3d, 4d, 5d, and 6d) have fewer.
The origin of the planar nodes becomes clear if we examine the wave equation which, for instance, includes an xy term in the case of the 7dxy orbital. When either x = 0 or y = 0, then there must be a node and this, by definition, is the case for the yz and xz planes.
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.