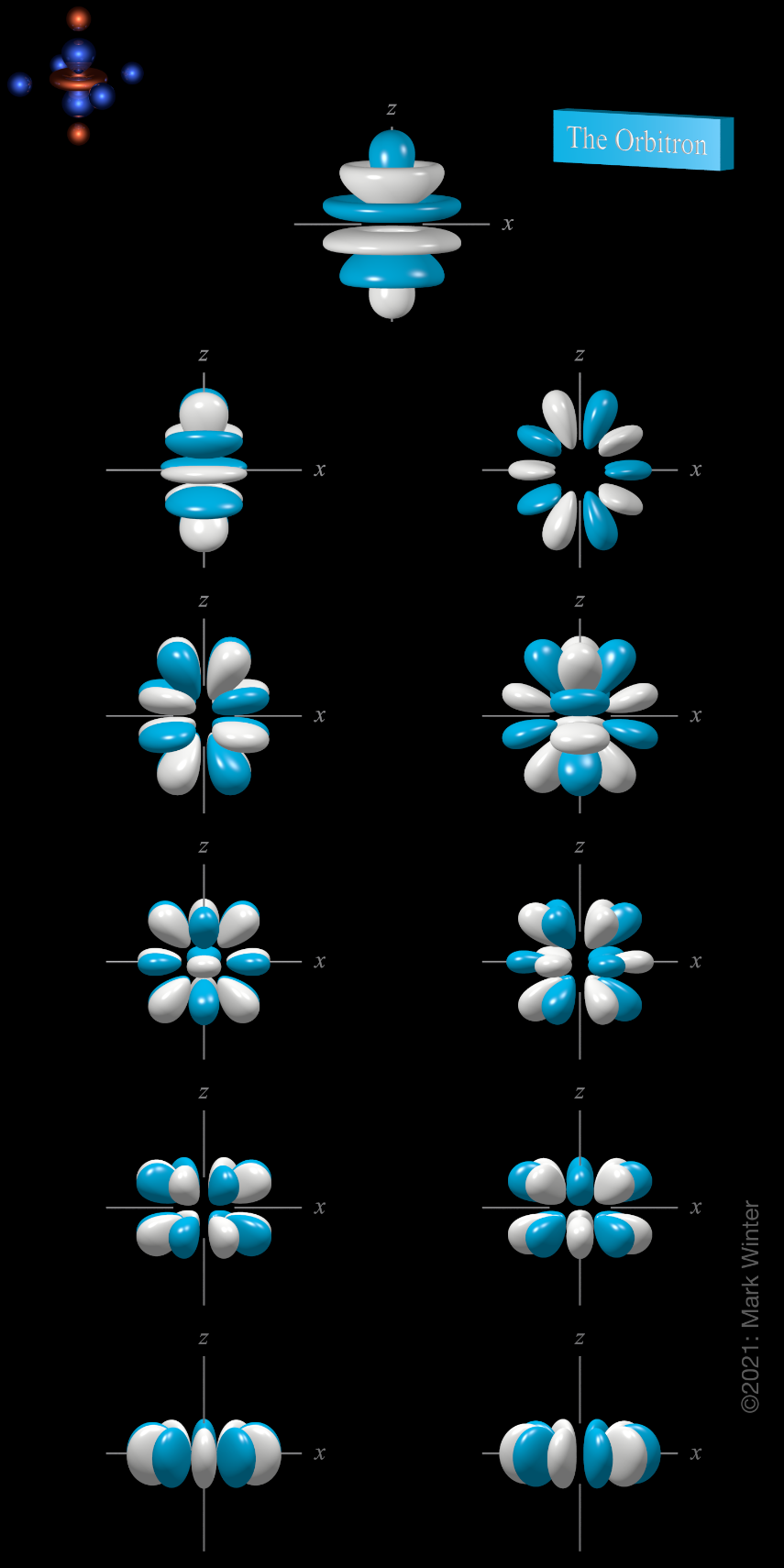
6h atomic orbitals
There are eleven 6h orbitals. These are labelled 6hz5, 6hyz4, 6hxz4, 6hz3xy, 6hz3(x2–y2), 6hz2y3, 6hz2x3, 6hz(4x3y–4xy3), 6hz(x4–6x2y2+y4), 6hyx4, and 6hxy4.
The shape of the 6h orbitals. Orbital names are shown beneath the images. For each, the turquoise (cyan) zones are where the values of the 6h wave functions are positive while the white zones denote negative values.
The 6hz5 orbital (top row in the image above) is an abbreviation for 5hz(63z4 - 70z2r2 + 15r4).
The 6hyz4 orbital (left orbital in next to top row in the above image) is an abbreviation for 6hy(21z4–14z2r2+r4). The 6hxz4 orbital (right orbital in next to top row in the above image) is an abbreviation for 6hx(21z4–14z2r2+r4). These two orbitals are related to each other by a 90° rotation about the z-axis.
The 6hz3xy orbital (left orbital in third row from the top in the above image) is an abbreviation for 6h(2xy)(3z3–zr2). The 6hz3(x2–y2) orbital (right orbital in third row from the top in the above image) is an abbreviation for 6h(x2–y3)(3z3–zr2). These two orbitals are related to each other by a 45° rotation about the z-axis.
The 6hz2y3 (left orbital in fourth row from the top in the above image) is an abbreviation for 6hy(3x2–y2)(9z2–r2). The 6hz2x3 orbital (right orbital in fourth row from the top in the above image) is an abbreviation for 6hx(x2–3y2)(9z2–r2). These two orbitals are related to each other by a 30° rotation about the z-axis.
The 6hz(4x3y–4xy3) orbital is the left orbital in fifth row from the top in the above image). The 6hz(x4–6x2y2+y4) orbital is the right orbital in fifth row from the top in the above image. These two orbitals are related to each other by a 22.5° rotation about the z-axis.
The 6hyx4 (left orbital in bottom row in the above image) is an abbreviation for 6hy(5x4–10y2x2+y4) and 6hxy4 (right orbital in bottom row in the above image) is an abbreviation for 6hx(x4–10y2x2+5y4). They are related to each other by a 18° rotation about the z-axis.
The 6h orbitals do not have spherical nodes but the higher horbitals (7h, 8h, 9h, ...) are more complex since they have spherical nodes.
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.