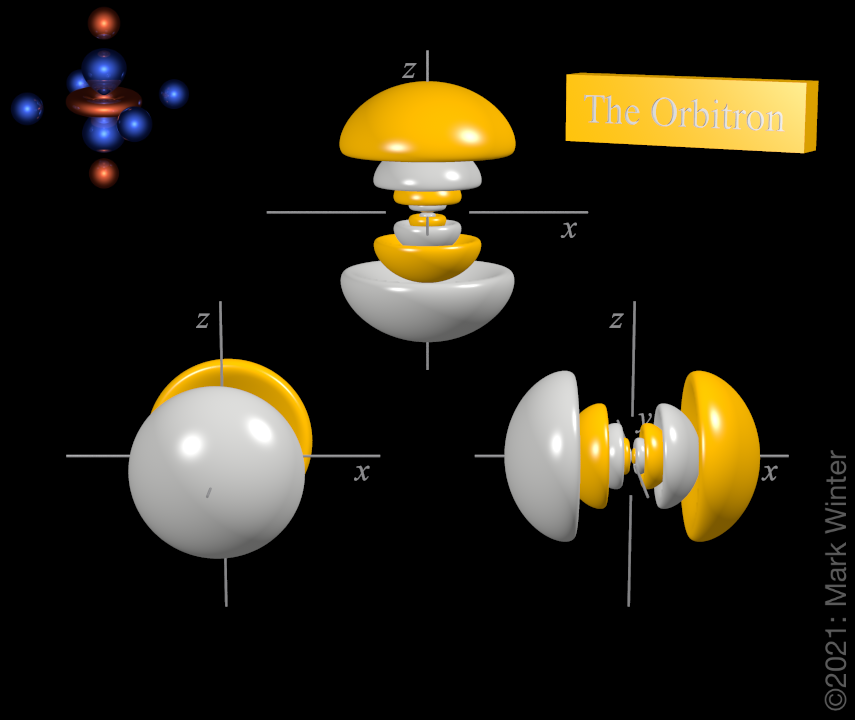
6p orbitals
The shape of the three 6p orbitals. From left to right: 6py, 6pz, and 6px. For each, the yellow zones are where the wave functions have positive values and the white zones denote negative values.
For any atom, there are three 6p orbitals. These orbitals have the same shape but are aligned differently in space. The three 6p orbitals normally used are labelled 6px, 6py, and 6pz since the functions are "aligned" along the x, y, and z axes respectively.
Each 6p orbital has ten lobes. There is a planar node normal to the axis of the orbital (so the 6px orbital has a yz nodal plane, for instance). Apart from the planar node there are also four spherical nodes that partition off the small inner lobes. The 7p) orbital is more complex still since it has five spherical nodes.
The origin of the planar node becomes clear when inspecting the wave equations which, for instance, includes an x term in the case of the 6px orbital. When x = 0, then there is a node, and this by definition is the yz plane.
The origin of the spherical nodes becomes clearer when inspecting the wave equations, which include a (840 - 840ρ + 252ρ2 - 28ρ3 + ρ4) term. When (840 - 840ρ + 252ρ2 - 28ρ3 + ρ4) = 0, then there are nodes.
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.