Atomic orbitals: 4d radial distribution function
Schematic plot of the 4d radial distribution function r2R4d2 (R4d = radial wave function).
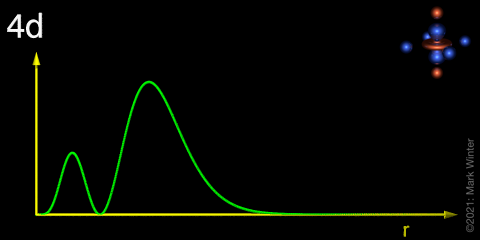
For 4d-orbitals, the radial distribution function is related to the product obtained by multiplying the square of the radial wave function R4d by r2. By definition, it is independent of direction.
In addition to two planar nodes (or two conical node in the case of the 4dz2 orbital), d-orbitals, display a number of radial nodes that separate the largest, outer, component from the inner components. The number of nodes is related to the principal quantum number, n. In general, the nd orbitals have (n - 3) radial nodes. Therefore, the 4d-orbitals each have (4 - 3) = 1 radial node, as shown in the above plot. Further radial nodes become evident in higher d-orbitals (5d, 6d, and 7d) but fewer nodes in lower d-orbitals (3d.
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.