Gadolinium - 64Gd: properties of free atoms
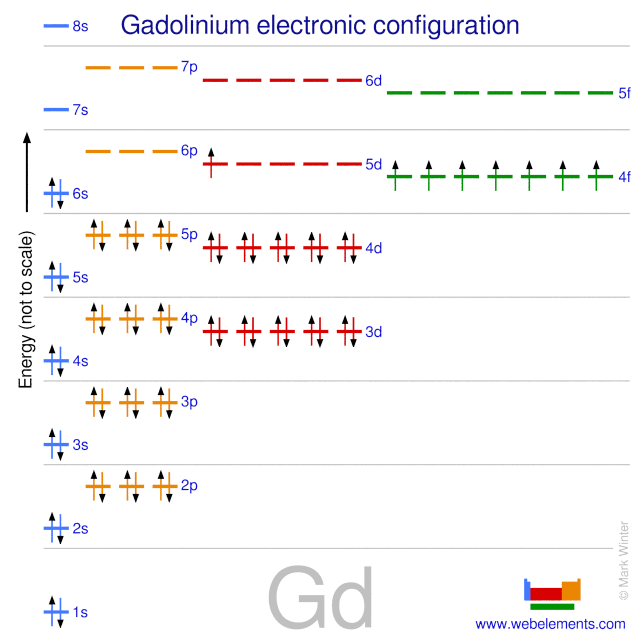
Gadolinium atoms have 64 electrons and the shell structure is 2.8.18.25.9.2.
The ground state electron configuration of ground state gaseous neutral gadolinium is [Xe].4f7.5d1.6s2 and the term symbol is 9D2.

Atomic spectrum
A representation of the atomic spectrum of gadolinium.
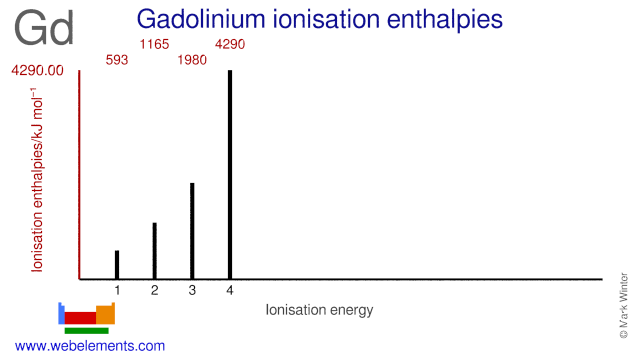
Ionisation Energies and electron affinity
The electron affinity of gadolinium is < 50 0.5 eV kJ mol‑1. The ionisation energies of gadolinium are given below.
Effective Nuclear Charges
The following are "Clementi-Raimondi" effective nuclear charges, Zeff. Follow the hyperlinks for more details and for graphs in various formats.
| 1s | 62.74 | ||||||
|---|---|---|---|---|---|---|---|
| 2s | 47.22 | 2p | 59.71 | ||||
| 3s | 43.71 | 3p | 44.15 | 3d | 50.28 | ||
| 4s | 33.44 | 4p | 32.65 | 4d | 29.63 | 4f | 25.01 |
| 5s | 18.88 | 5p | 16.76 | 5d | (no data) | ||
| 6s | 8.21 | 6p | (no data) | ||||
| 7s | |||||||
References
These effective nuclear charges, Zeff, are adapted from the following references:
- E. Clementi and D.L.Raimondi, J. Chem. Phys. 1963, 38, 2686.
- E. Clementi, D.L.Raimondi, and W.P. Reinhardt, J. Chem. Phys. 1967, 47, 1300.
Electron binding energies
| Label | Orbital | eV [literature reference] |
|---|---|---|
| K | 1s | 50239 [1] |
| L I | 2s | 8376 [1] |
| L II | 2p1/2 | 7930 [1] |
| L III | 2p3/2 | 7243 [1] |
| M I | 3s | 1881 [1] |
| M II | 3p1/2 | 1688 [1] |
| M III | 3p3/2 | 1544 [1] |
| M IV | 3d3/2 | 1221.9 [2] |
| M V | 3d5/2 | 1189.6 [2] |
| N I | 4s | 378.6 [2] |
| N II | 4p1/2 | 286 [1] |
| N III | 4p3/2 | 271 [1] |
| N IV | 4d3/2 | - |
| N V | 4d5/2 | 142.6 [2] |
| N VI | 4f5/2 | 8.6 [2] |
| N VII | 4f7/2 | 8.6 [2] |
| O I | 5s | 36 [1] |
| O II | 5p1/2 | 20 [1] |
| O III | 5p3/2 | 20 [1] |
Notes
I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data. The data are adapted from references 1-3. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5).
References
- J. A. Bearden and A. F. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. Mod. Phys., 1967, 39, 125.
- M. Cardona and L. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978.
- Gwyn Williams WWW table of values
- D.R. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000.
- J. C. Fuggle and N. Mårtensson, "Core-Level Binding Energies in Metals," J. Electron Spectrosc. Relat. Phenom., 1980, 21, 275.