Argon - 18Ar: properties of free atoms
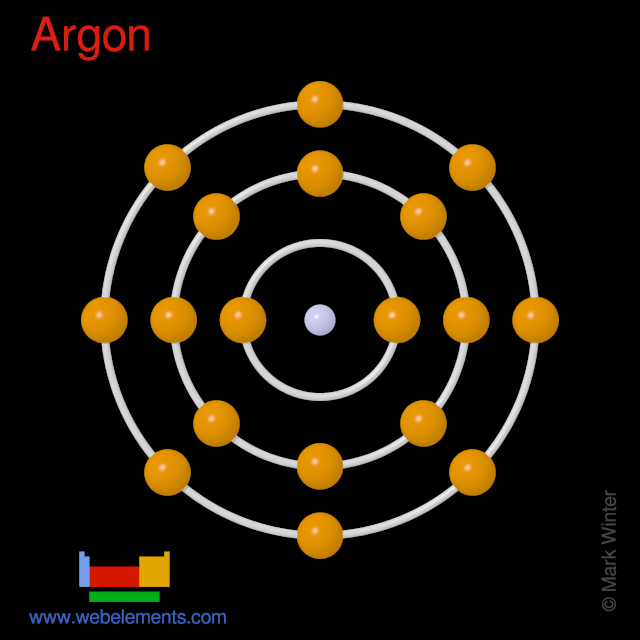
Argon atoms have 18 electrons and the shell structure is 2.8.8.
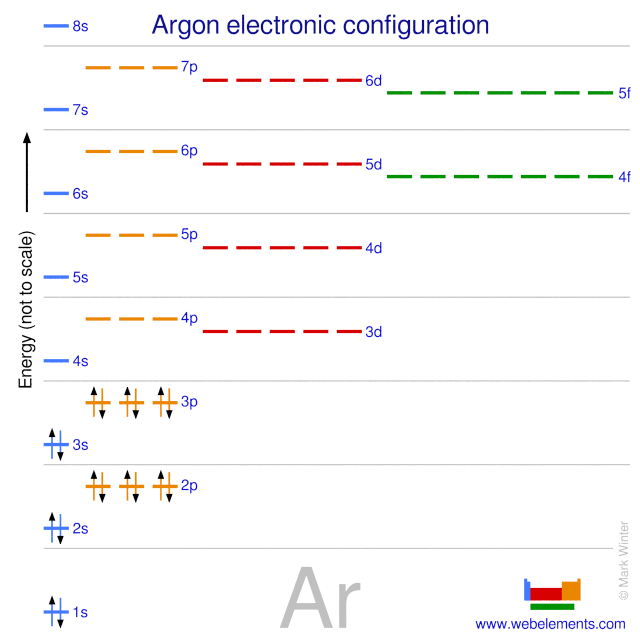
The ground state electron configuration of ground state gaseous neutral argon is [Ne].3s2.3p6 and the term symbol is 1S0.
Atomic spectrum
A representation of the atomic spectrum of argon.
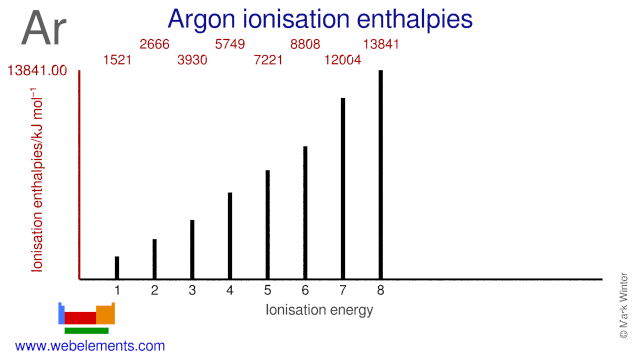
Ionisation Energies and electron affinity
The electron affinity of argon is < 0 0 eV kJ mol‑1. The ionisation energies of argon are given below.
Effective Nuclear Charges
The following are "Clementi-Raimondi" effective nuclear charges, Zeff. Follow the hyperlinks for more details and for graphs in various formats.
| 1s | 17.5075 | ||||||
|---|---|---|---|---|---|---|---|
| 2s | 12.23 | 2p | 14.01 | ||||
| 3s | 7.76 | 3p | 6.76 | 3d | (no data) | ||
| 4s | (no data) | 4p | (no data) | 4d | (no data) | 4f | (no data) |
| 5s | (no data) | 5p | (no data) | 5d | (no data) | ||
| 6s | (no data) | 6p | (no data) | ||||
| 7s | |||||||
References
These effective nuclear charges, Zeff, are adapted from the following references:
- E. Clementi and D.L.Raimondi, J. Chem. Phys. 1963, 38, 2686.
- E. Clementi, D.L.Raimondi, and W.P. Reinhardt, J. Chem. Phys. 1967, 47, 1300.
Electron binding energies
| Label | Orbital | eV [literature reference] |
|---|---|---|
| K | 1s | 3205.9 [2] |
| L I | 2s | 326.3 [2] |
| L II | 2p1/2 | 250.6 [3] |
| L III | 2p3/2 | 248.4 [2] |
| M I | 3s | 29.3 [2] |
| M II | 3p1/2 | 15.9 [2] |
| M III | 3p3/2 | 15.7 [2] |
Notes
I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data. The data are adapted from references 1-3. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5).
References
- J. A. Bearden and A. F. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. Mod. Phys., 1967, 39, 125.
- M. Cardona and L. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978.
- Gwyn Williams WWW table of values
- D.R. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000.
- J. C. Fuggle and N. Mårtensson, "Core-Level Binding Energies in Metals," J. Electron Spectrosc. Relat. Phenom., 1980, 21, 275.