| The University of Sheffield |
| Department of Chemistry |
VSEPR |
Radicals and VSEPR calculation for nitrogen dioxide, NO2
Another complication crops up when there are unpaired electrons. This is well illustrated for nitrogen dioxide. So, for NO2 there is an integral number of electrons but a non-integral number of electron pairs. Since any orbital can accommodate 0, 1, or 2 electrons, 21/2 electron pairs must be placed into three orbitals. Therefore the geometry is based upon a trigonal-planar arrangement of electron pairs. Since the lone-pair orbital is only half filled, it demands less space, and the O-N-O angle opens out a little (to 134.1°) from the ideal trigonal angle of 120°.
Addition of one electron to NO2 gives the nitrite anion NO2-. This last electron completes the half-occupied lone-pair orbital and this filling of the orbital causes it to fill out, and so close the O-N-O bond angle to 115°.
Nitrogen dioxide, NO2
| Lewis structure: |
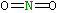 |
| Central atom |
nitrogen |
| Valence electrons on central atom: |
5 |
| 2 terminal oxygens each contribute 1 electron in the two σ bonds: |
2 |
| Subtract two for the two electrons contributed by N to the two π bonds: |
-2 |
| Total: |
6 |
| Divide by 2 to give electron pairs |
21/2 |
| 21/2 electron pairs: |
trigonal geometry for the 21/2 shape-determining σ-framwork orbitals |
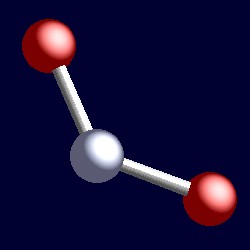 |
|
| The geometry of nitrogen dioxide, NO2. You can use your mouse to manipulate the molecule in the right hand "Jmol" image. |
| 

