| The University of Sheffield |
| Department of Chemistry |
VSEPR |
VSEPR calculation for boron trifluoride, BF3
Boron trifluoride only has six valence electrons and is one of the relatively rare second period covalent molecules that disobeys the octet rule. There are three bonded groups and so no lone pairs. Six electrons implies three electron pairs and therefore a trigonal geometry.
Boron trifluoride, BF3
| Lewis structure: |
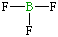 |
| Central atom: |
boron |
| Valence electrons on central atom: |
3 |
| 3 F each contribute 1 electron: |
3 |
| Total: |
6 |
| Divide by 2 to give electron pairs |
3 |
| 3 electron pairs: |
trigonal geometry for the three shape-determining electron pairs |
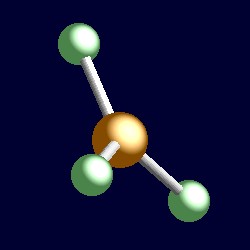 |
|
| The geometry of boron trifluoride, BF3. You can use your mouse to manipulate the molecule in the right hand "Jmol" image. |
| 

