| The University of Sheffield |
| Department of Chemistry |
VSEPR |
VSEPR calculation for trifluorothionitrile, SF3N
Note that the net effect of a triple bonded group such as ≡N is -1, made up from +1 in the σ bond and -2 for the two π bonds. As for a double bond, the S≡N bond takes up more room than a single bond and this causes the F3S≡N F-S-F bond to drop a little below the ideal tetrahedral bond angle.
Trifluorothionitrile, F3S≡N
| Lewis structure: |
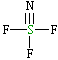 |
| Central atom: |
sulphur |
| Valence electrons on central atom: |
6 |
| 3 F atoms each contribute 1 electron: |
3 |
| 1 terminal nitrogen contributes 1 electron in a σ bond |
1 |
| Subtract two for the two electrons contributed by S to the two π bonds |
-2 |
| Total: |
8 |
| Divide by 2 to give electron pairs |
4 |
| 4 electron pairs: |
tetrahedral geometry for the four shape-determining electron pairs |
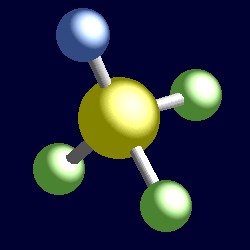 |
|
| The geometry of trifluorothionitrile, F3S≡N. You can use your mouse to rotate the molecule in the right hand "JMol" image. |
|