| The University of Sheffield |
| Department of Chemistry |
VSEPR |
VSEPR calculation for methane, CH4
The calculation for methane shows that the carbon atom is associated with 8 electrons in the σ framework. This corresponds to four shape-determining electron pairs. The coordination geometry of carbon is consequently tetrahedral. There are four bonded groups, therefore there are no lone pairs.
Methane (CH4)
| Lewis structure: |
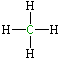 |
| Central atom: |
carbon |
| Valence electrons on central atom: |
4 |
| 4 H each contribute 1 electron: |
4 |
| Total: |
8 |
| Divide by 2 to give electron pairs |
4 |
| 4 electron pairs: |
tetrahedral for the four shape-determining electron pairs |
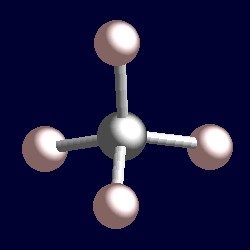 |
|
| The geometry of methane, CH4. You can use your mouse to manipulate the molecule in the right hand "Jmol" image. |
| 


