| The University of Sheffield |
| Department of Chemistry |
VSEPR |
VSEPR calculation for propene, CH3CH=CH2
For propene the calculation again predicts no lone pairs on the central carbon. Here the VSEPR treatment is seen for a double bond, and note the VSEPR method works for organic species just as well as it does for inorganic molecules. The π electrons in the double bond cause that vertex to appear a little bigger than a simple σ bond and so to make room, the Me-C=CH2 bond angle (124.8°) is a little greater than the ideal angle of 120°.
Propene, MeCH=CH2
| Lewis structure: |
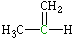 |
| Central atom: |
Carbon (MeCH=CH2) |
| Valence electrons on central atom: |
4 |
| 1 Me group contributes 1 electron: |
1 |
| 1 H atom contributes 1 electron |
1 |
| 1 =CH2 group contributes 1 electron in a σ bond |
1 |
| Subtract one for the electron contributed by C to the π bond |
-1 |
| Total |
6 |
| Divide by 2 to give electron pairs |
3 |
| 3 electron pairs: |
trigonal geometry for the three shape-determining electron pairs |
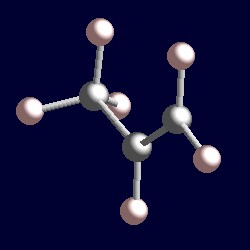 |
| |
| The geometry of propene, MeCH=CH2. You can use your mouse to manipulate the molecule in the right hand "Jmol" image. |
| 

