| The University of Sheffield |
| Department of Chemistry |
VSEPR |
VSEPR calculation for water, OH2
Water has four electron pairs and the coordination geometry of oxygen is based upon a tetrahedral arrangement of electron pairs. Since there are only two bonded groups, there are two lone pairs. Since the lone pairs are not 'seen', the shape of water is bent. The two lone pairs compress the H-O-H bond angle below the ideal tetrahedral angle to 104.5°.
Water, OH2
| Lewis structure: |
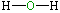 |
| Central atom: |
oxygen |
| Valence electrons on central atom: |
6 |
| 2 H each contribute 1 electron: |
2 |
| Total: |
8 |
| Divide by 2 to give electron pairs |
4 |
| 4 electron pairs: |
tetrahedral for the four shape-determining electron pairs |
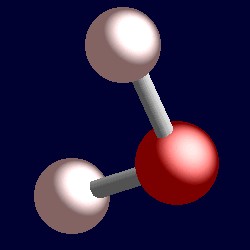 |
|
| The geometry of water, OH2. You can use your mouse to manipulate the molecule in the right hand "JMol" image. |
| 

