| The University of Sheffield |
| Department of Chemistry |
VSEPR |
VSEPR calculation for hexafluorophosphate, [PF6]-
For hexafluorophosphate, [PF6]-, there are six bonded groups and so no lone pairs. This anion is useful in synthesis since it often aids the crystallization of bulky cations by providing a reasonable size match for the cation. Note that the negative charge for the purposes of the calculation is placed on phosphorus for the purpose of the calculation even though the negative charge is in reality delocalized over all seven atoms of the ion.
hexafluorophosphate, [PF6]-
| Lewis structure: |
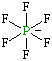 |
| Central atom: |
phosphorus |
| Valence electrons on central atom: |
5 |
| 6 F each contribute 1 electron: |
6 |
| Add one for the negative charge on P |
1 |
| Total: |
12 |
| Divide by 2 to give electron pairs |
6 |
| 6 electron pairs: |
octahedral geometry for the six shape-determining electron pairs |
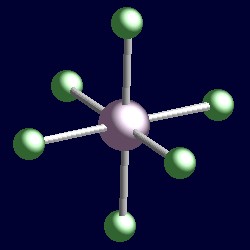 |
|
| The geometry of hexafluorophosphate, [PF6]-. You can use your mouse to manipulate the molecule in the right hand "Jmol" image. |
| 


