 |
|---|
| VSEPR home |
|
|
| The University of Sheffield | |
| Department of Chemistry | VSEPR |
VSEPR calculation for ethanoate (acetate), MeCO2-
| Lewis structure: | |
| Central atom | carbon |
| Valence electrons on central atom: | 4 |
| 1 Me group contributes 1 electron: | 1 |
| 2 terminal oxygens each contribute 1 electron in the two σ bonds: | 2 |
| Subtract two for the two electrons contributed by C to the two π bonds | -2 |
| Add one for the negative charge located on C | 1 |
| Total: | 6 |
|---|---|
| Divide by 2 to give electron pairs | 3 |
| 3 electron pairs: | trigonal geometry for the three shape-determining electron pairs |
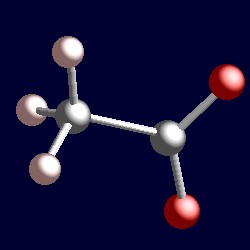 |
|
|---|---|
| The geometry of ethanoate (acetate), MeCO2-. You can use your mouse to manipulate the molecule in the right hand "Jmol" image. | |
 |
|---|
|
|