| The University of Sheffield |
| Department of Chemistry |
VSEPR |
VSEPR calculation for H3N→BF3
Since BF3 is deficient two electrons from the octet, a characteristic reaction is to react with lone pair donors. Acceptance of a pair of electrons makes BF3 a Lewis acid. The electron pair donor NH3 is a Lewis base. The product of the reaction between NH3 and BF3 is sometimes written H3N→BF3. There are two atoms of interest, N and B. As in any other molecule containing more than one 'central' atom, it is necessary to execute a calculation for each centre.
H3N→BF3
| Lewis structure: |
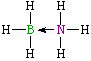 |
| Central atom: |
boron |
| Valence electrons on central atom: |
3 |
| 3 F each contribute 1 electron: |
3 |
| NH3 donates 2 electrons in dative bond: |
2 |
| Total: |
8 |
| Divide by 2 to give electron pairs |
4 |
| 4 electron pairs: |
tetrahedral geometry for the four shape-determining electron pairs |
| |
| Central atom |
Nitrogen |
| Valence electrons on central atom: |
5 |
| 3 H each contribute 1 electron: |
3 |
| BF3 donates 0 electrons in dative bond |
0 |
| Total: |
8 |
| Divide by 2 to give electron pairs |
4 |
| 4 electron pairs: |
tetrahedral geometry for the four shape-determining electron pairs |
 |
|
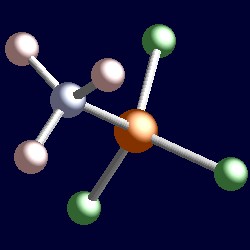
| The geometry of H3N→BF3. You can use your mouse to manipulate the molecule in the right hand "Jmol" image. |
An alternative representation (below) of BF3NH3 places a positive charge on nitrogen and a negative charge on fluorine. The octet rule is still satisfied for each atom and it is now not necessary to write in an arrow to distinguish the B-N bond from other single bonds. In some ways this is an advantage since it removes the impression that a dative bond is different from other bonds. The VSEPR calculation works perfectly well on this representation.
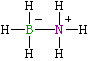
|