Sulfur - 16S: biological information
Sulphur is essential to life. It is a minor constituent of fats, body fluids, and skeletal minerals. Sulphur is a key component in most proteins since it is contained in the amino acids methionine and cysteine. Sulphur-sulphur interactions are important in determining protein tertiary structure. Hydrogen sulphide (H2S) replaces H2O in the photosynthesis of some bacteria. In people, hydrogen sulphide in very small concentrations can be metabolized, but in higher concentrations it kills quickly by preventing respiration. It is insidious in that it deadens the sense of smell quickly, meaning victims may be unaware of its presence. It is more toxic than cyanide. Remarkably, sulphuric acid (H2SO4) is present in the digestive fluids of sea squirts (ascidians).
Levels in humans
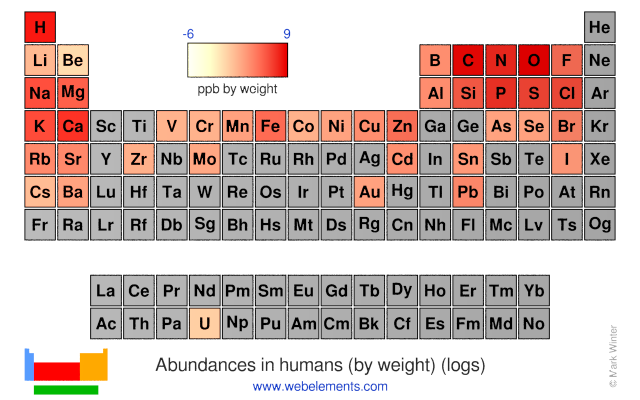
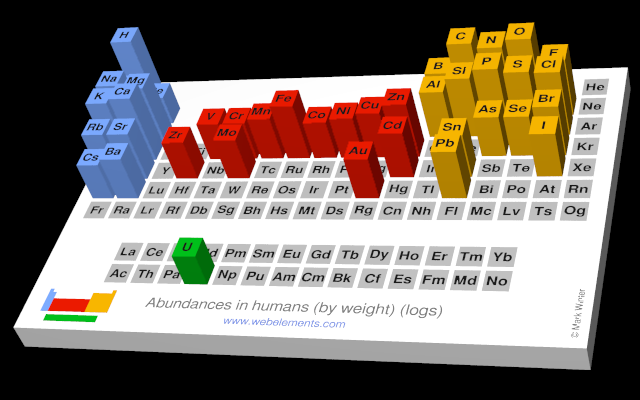
The abundances of the elements in humans.
- Human abundance by weight: 2000000 ppb by weight
- Human abundance by atoms: 390000 atoms relative to C = 1000000
How much sulfur is in your body? Find out here.
You can use this form to calculate how much sulfur your body contains. Enter your weight in either kilograms or pounds and click the "Calculate" button. You must enter a number, not text! Elements for which there are no data will always give a value of zero for the weight, no matter what you put in the weight box.
Hazards and Risks
Hazards and risks associated with sulfur:
Elemental sulphur is relatively harmless, but is very toxic to many bacteria and fungi. Sulphur dust irritates eyes and eyelids.
Carbon disulphide, hydrogen sulphide, and sulphur dioxide should be handled extremely carefully. Hydrogen sulphide in very small concentrations can be metabolised, but in higher concentrations it can cause death quickly by respiratory paralysis. It is insidious in that it quickly deadens the sense of smell and is more toxic than cyanide. Sulphur dioxide is a dangerous component in atmospheric air pollution and is one of the factors responsible for acid rain.
Carbon disulphide, CS2, is an important industrial solvent. It must be handled carefully since it is poisonous. It is easily absorbed through skin and by inhalation. It causes problems to the central nervous system.
References
- J.E. Huheey, E.A. Keiter, and R.L. Keiter in Inorganic Chemistry : Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.
- S. Budavari (Ed.) in The Merck Index, 11th ed., Merck, USA, 1989.
- N.N. Greenwood and A. Earnshaw in Chemistry of the Elements, 2nd edition, Butterworth, UK, 1997.