Fluorine - 9F: biological information
Fluorine as fluoride (F-) is probably an essential element for humans and certainly is for some molluscs. In some areas, fluoride ion is added to drinking water (in very low concentrations) since it renders tooth enamel relatively immune to bacteriological attack. It does this by replacing the OH group of hydroxyapatite with fluoride. In other areas, fluoride is not added to water, despite the benefits, as a consequence of protests from civil rights activists who object to the addition of anything to water.
Levels in humans
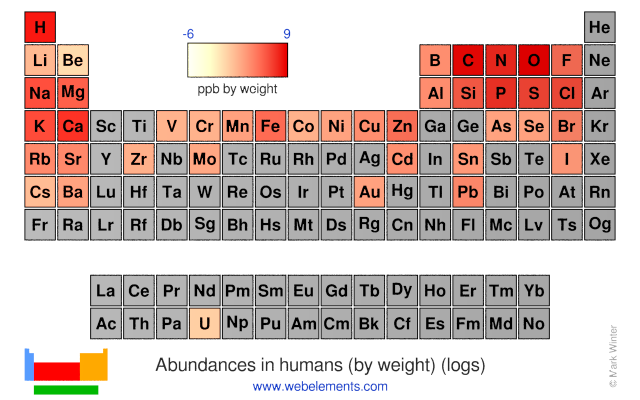
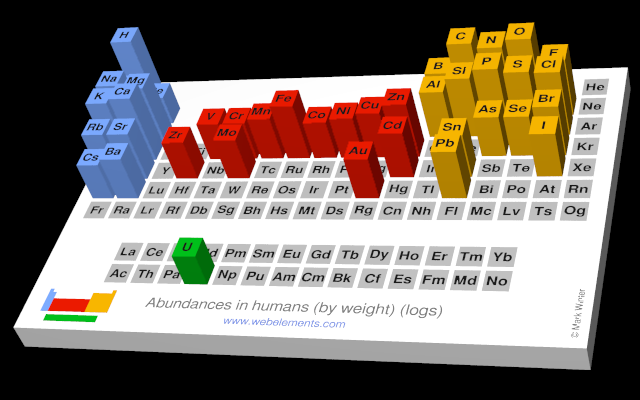
The abundances of the elements in humans.
- Human abundance by weight: 37000 ppb by weight
- Human abundance by atoms: 12000 atoms relative to C = 1000000
How much fluorine is in your body? Find out here.
You can use this form to calculate how much fluorine your body contains. Enter your weight in either kilograms or pounds and click the "Calculate" button. You must enter a number, not text! Elements for which there are no data will always give a value of zero for the weight, no matter what you put in the weight box.
Hazards and Risks
Hazards and risks associated with fluorine:
Fluorine gas is extremely corrosive and toxic. The free element has a characteristic pungent odour, detectable in concentrations as low as 20 ppb, which is below the safe working level. Exposure to low concentrations causes eye and lung irritation. Metal fluorides are very toxic. Organic fluorides are generally much less toxic and are often quite harmless. Fluoride ion is a very useful component of drinking water (in very low concentrations) since it renders tooth enamel (replacing the OH group of hydroxyapatite) relatively immune to bacteriological attack. However in some areas, fluoride is not added to water, despite the benefits, as a consequence of civil rights activists who object to the addition of anything to water.
References
- J.E. Huheey, E.A. Keiter, and R.L. Keiter in Inorganic Chemistry : Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.
- S. Budavari (Ed.) in The Merck Index, 11th ed., Merck, USA, 1989.
- N.N. Greenwood and A. Earnshaw in Chemistry of the Elements, 2nd edition, Butterworth, UK, 1997.