Sodium - 11Na: geological information
Sodium is never found as the free element ("native") in nature as it is so reactive. Sodium is the sixth most abundant element in the earth's crust at about 2.6 - 3.0%. The most common mineral is rock salt (sodium chloride, NaCl, or halite), but it occurs in many other minerals including sodium borate (borax), sodium carbonate (soda), sodium nitrate (Chile saltpetre). and sodium sulphate (thenardite). In those species, however, it is the anions that are the reason for mining.
Sodium is present in some abundance in the sun and other stars andclearly identifiable by the sodium D lines which are very prominent in the solar spectrum and those of other stars.
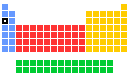
Abundances of sodium in various environments
In this table of abundances, values are given in units of ppb (parts per billion; 1 billion = 109), both in terms of weight and in terms of numbers of atoms. Values for abundances are difficult to determine with certainty, so all values should be treated with some caution, especially so for the less common elements. Local concentrations of any element can vary from those given here an orders of magnitude or so and values in various literature sources for less common elements do seem to vary considerably.
| Location | ppb by weight | ppb by atoms |
|---|---|---|
| Universe | 20000 | 1000 |
| Sun | 40000 | 2000 |
| Meteorite (carbonaceous) | 5600000 | 4800000 |
| Crustal rocks | 23000000 | 21000000 |
| Sea water | 11050000 | 2970000 |
| Stream | 8000 | 350 |
| Human | 1400000 | 380000 |
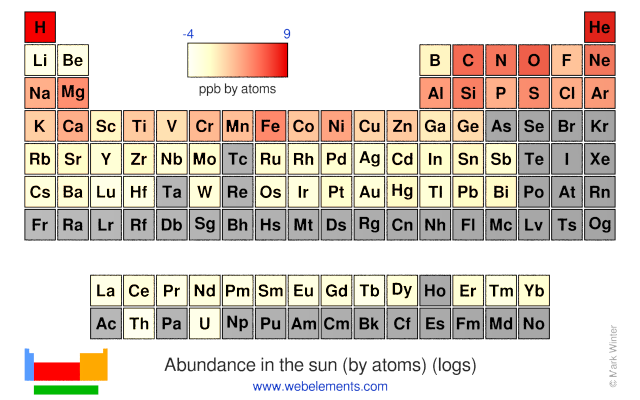
The chart above shows the log of the abundance (on a parts per billion scale) of the elements by atom number in our sun. Notice the "sawtooth" effect where elements with even atomic numbers tend to be more strongly represented than those with odd atomic numbers. This shows up best using the "Bar chart" option on the chart.