Nitrogen - 7N: thermochemistry and thermodynamics
Temperatures
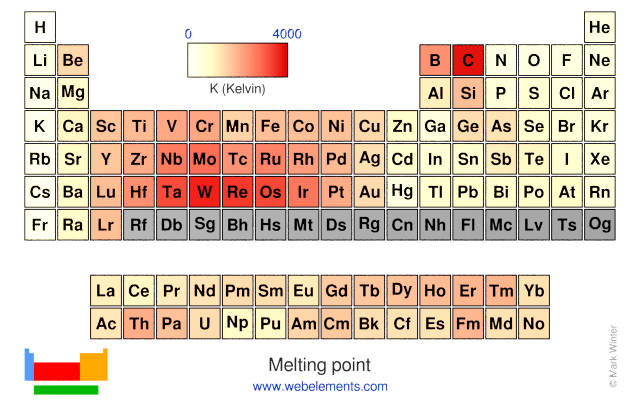
 Melting point: 63.05 [‑210.1 °C (‑346.18 °F)] K
Melting point: 63.05 [‑210.1 °C (‑346.18 °F)] K Boiling point: 77.36 [‑195.79 °C (‑320.42 °F)] K
Boiling point: 77.36 [‑195.79 °C (‑320.42 °F)] K Liquid range: 14.31 K
Liquid range: 14.31 K  Critical temperature: 126.2 [‑146.9 °C (‑232.4 °F)] K
Critical temperature: 126.2 [‑146.9 °C (‑232.4 °F)] K Superconduction temperature: (no data) K
Superconduction temperature: (no data) K
Enthalpies
 Enthalpy of fusion: 0.36 (per mol N atoms) kJ mol-1
Enthalpy of fusion: 0.36 (per mol N atoms) kJ mol-1 Enthalpy of vaporisation: 2.79 (per mole N atoms) kJ mol-1
Enthalpy of vaporisation: 2.79 (per mole N atoms) kJ mol-1 Enthalpy of atomisation: 473 kJ mol-1
Enthalpy of atomisation: 473 kJ mol-1
Thermodynamic data
This table gives a few thermodynamic data for nitrogen. Most values are those given in the NBS technical notes (reference 1) after conversion from the units used within those notes. Values labelled with an asterisk (*) are Committee on Data for Science and Technology (CODATA) agreed values for the thermodynamic properties of key chemical substances (reference 2). These values are published in a number of places including the WWW (reference 3).
| State | ΔfH° | ΔfG° | S° | CpH | H°298.15‑H°0 |
|---|---|---|---|---|---|
| Units | kJ mol‑1 | kJ mol‑1 | J K‑1 mol‑1 | J K‑1 mol‑1 | kJ mol‑1 |
| Gas (N2) | *0 | 0 | *191.609 ± 0.004 | 29.12 | *8.670 ± 0.001 |
| Gas (atoms) | *472.68 ± 0.40 | 455.58 | *153.301 ± 0.003 | 20.79 | *6.197 ± 0.001 |
Notes
This tables gives a few thermodynamic data. Most values are those given in the NBS technical notes (reference 1) after conversion from the units used within those notes. Values labelled with an asterisk (*) are Committee on Data for Science and Technology (CODATA) agreed values for the thermodynamic properties of key chemical substances (reference 2). These values are published in a number of places including the WWW (reference 3).
References
- R.H. Schumm, D.D. Wagman, S. Bailey, W.H. Evans, and V.B. Parker in National Bureau of Standards (USA) Technical Notes 270-1 to 270-8, 1973.
- http://www.codata.info/resources/databases/key1.html
- J.D. Cox, DD., Wagman, and V.A. Medvedev, CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp., New York, USA, 1989.