Iron - 26Fe: electronegativity
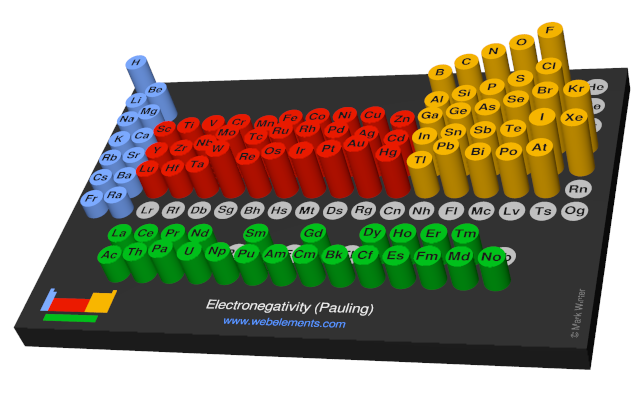
The most used definition of electronegativity is that an element's electronegativity is the power of an atom when in a molecule to attract electron density to itself. The electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. The first scale of electronegativity was developed by Linus Pauling and on his scale iron has a value of 1.83 on a scale running from from about 0.7 (an estimate for francium) to 2.20 (for hydrogen) to 3.98 (fluorine). Electronegativity has no units but "Pauling units" are often used when indicating values mapped on to the Pauling scale.
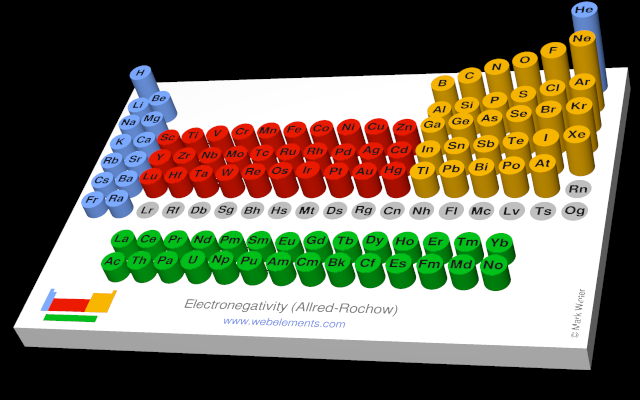
Use the links in the "Electronegativity" column of the table below for definitions, literature sources, and visual representations in several different styles (examples of which are shown below).
| Electronegativity | Value in Pauling units | Periodicity link |
|---|---|---|
| Pauling electronegativity | 1.83 |  |
| Sanderson electronegativity | 2.20 |  |
| Allred Rochow electronegativity | 1.64 |  |
| Mulliken-Jaffe electronegativity | (no data) |  |
| Allen electronegativity | 1.80 |  |
There are a number of ways to produce a set of numbers representing electronegativity and five are given in the table above. The Pauling scale is perhaps the most famous and suffices for many purposes.