Atomic orbitals: 6g electron "dot-density"
This page shows representations of electron density in two ways. The first is two-dimensional electron "dot-density" diagrams - plots across an appropriate plane of each 6g orbital. These were created using a Monte Carlo computational method. The second page allows you interact (zoom, rotate) with three-dimensional electron "dot-density" models representing electron density of the various 6g atomic orbitals created by the same Monte Carlo computational method. Green represents regions for which the wave functions are positive and white represents where values are negative.
6gz4 electron "dot-density"
The 6gz4 orbital is an abbreviation for 6g35z4 - 30z2r2 + 3r4.
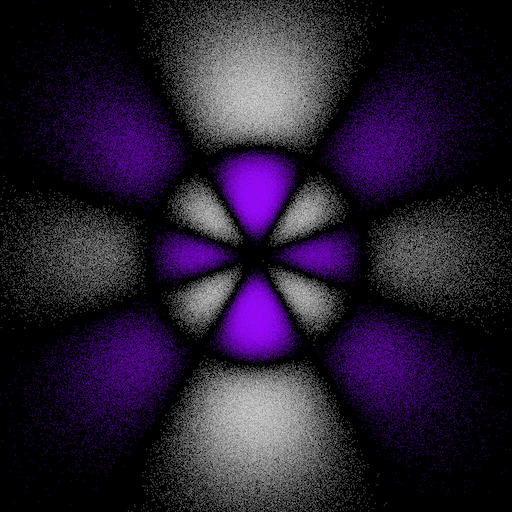
6gz3y and 6gz3x electron "dot-density"
The 6gz3x orbital is an abbreviation for 6gyz(7z2 - 3r2). The 6gz3y orbital is an abbreviation for 6gxz(7z2 - 3r2). These two orbitals are related to each other by a 90° rotation about the z-axis.
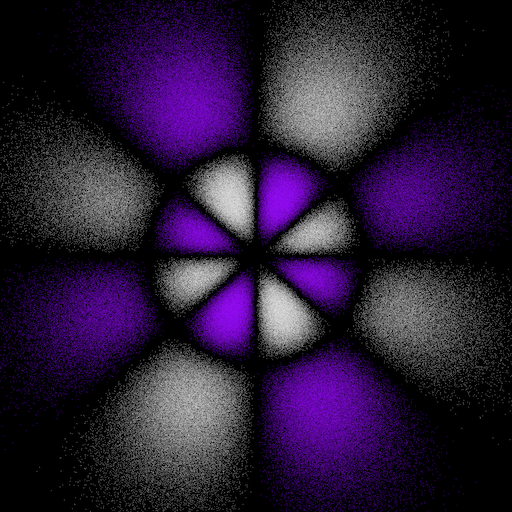
6gz2xy and 6gz2(x2-y2) electron "dot-density"
The 6gz2xy orbital is an abbreviation for 6gxy(7z2 - r2). The 6gz2(x2 - y2) orbital is an abbreviation for 6g(x2 - y2)(7z2 - r2). These two orbitals are related to each other by a 45° rotation about the z-axis.
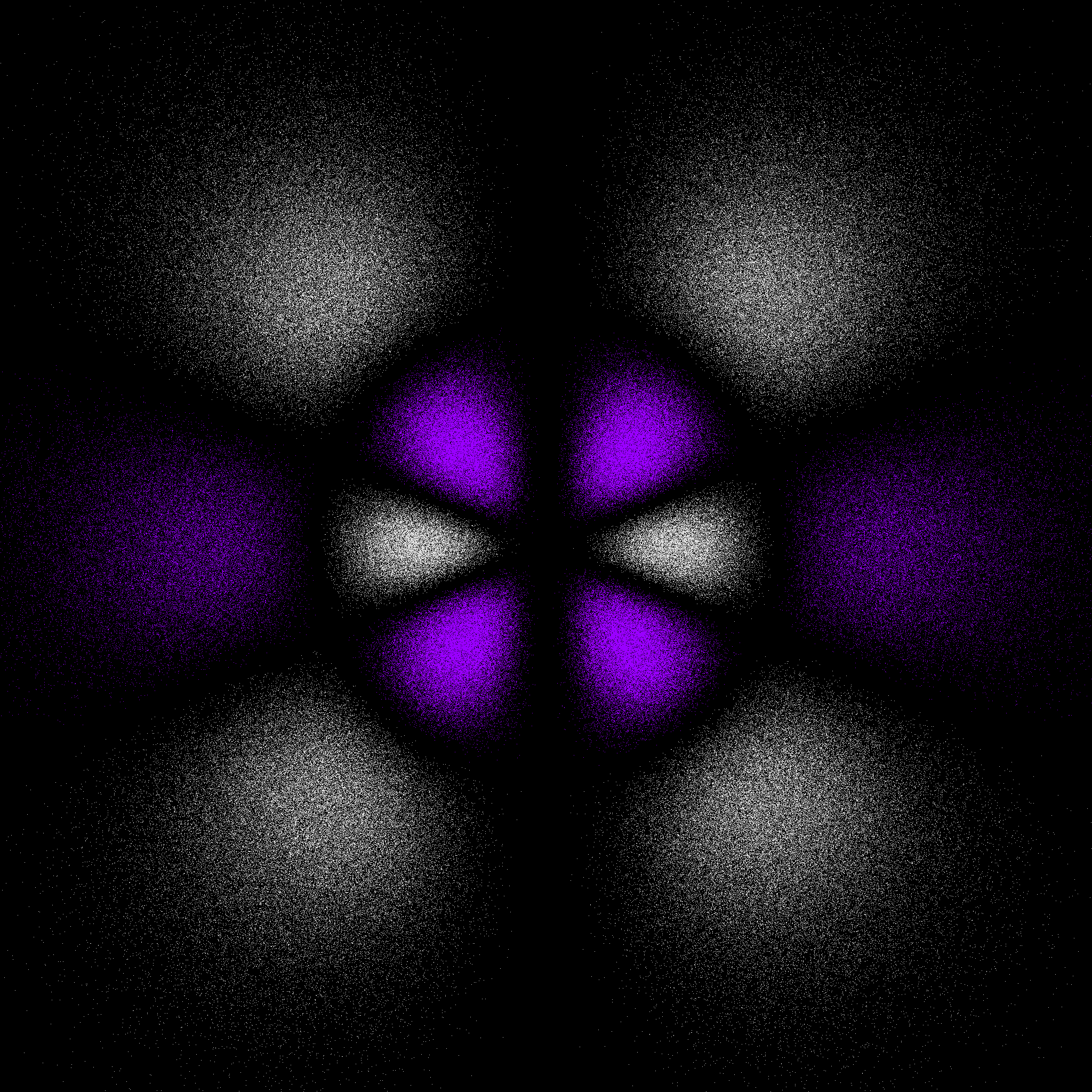
6gzy3 and 6gzx3 electron "dot-density"
The 6gzy3 is an abbreviation for 6gyz(3x2 - y2). The 6gzx3 orbital is an abbreviation for 6gxz(x2 - 3y2). These two orbitals are related to each other by a 30° rotation about the z-axis.
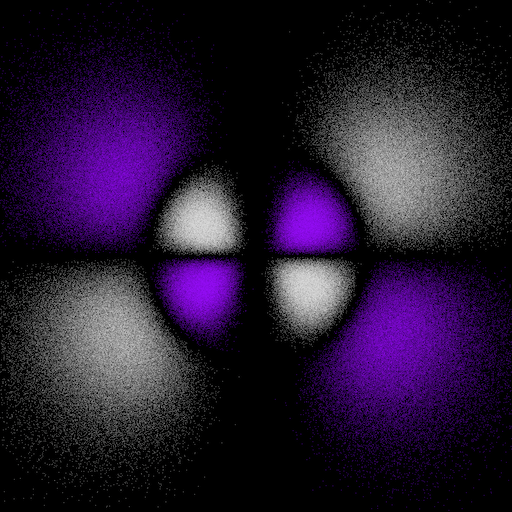
6gxy(x2-y2) and 6g(x4+y4) electron "dot-density"
The 6gxy(x2-y2) and 6g(x4+y4) orbitals are related to each other by a 22.5° rotation about the z-axis.
Using JSmol
You can use your mouse to manipulate the "orbital" in the "JSmol" image above. How you do this depends upon how you are viewing this page.
- On a computer, to rotate about the x and y directions, drag mouse around the image
- On a computer, to rotate about the z direction (which comes out of the screen towards you), hold shift and drag mouse horizontally
- To zoom, hold shift key down and drag mouse vertically
- To reset the image, hold down shift key and double click ony part of the image not containing dots
- On a mobile device experiment using one or two fingers to rotate and zoom
The OrbitronTM, a gallery of orbitals on the WWW: https://winter.group.shef.ac.uk/orbitron/
Copyright 2002-2023 Prof. Mark Winter [The University of Sheffield]. All rights reserved.